Developing new drugs for any disease is an expensive, time-consuming, and risky endeavor. This is especially true for central nervous system (CNS) disorders due to the difficulty of identifying and validating drug targets and getting sufficient amounts of the drug candidate into the brain to test its effectiveness. However, recent advances in nucleic acid sequencing, computation, and biotechnology are poised to revolutionize the treatment of neurological and psychiatric disorders.
Highlighting the contributions made to the field by Paul J. Kenny, PhD, and Yizhou Dong, PhD
High-throughput sequencing has enabled large-scale human genetics initiatives to uncover the genomic architectures of neuropsychiatric disorders—those variations in an individual’s genome sequence that confer risk or resistance for a given condition. Newer sequencing technologies have made it possible to profile the transcriptomes of brain cells with remarkable spatiotemporal and single-cell resolution (see Genetics and Epigenetics). These advances are identifying the biological pathways disrupted in neuropsychiatric disorders. With this knowledge, new drugs that target these same pathways are being developed, promising more effective treatment options.
We are identifying the biological pathways disrupted in neuropsychiatric disorders …
… and new drugs that target these same pathways are being developed.
Advances in neuropsychiatric genetics and stem cell biology are also leading to improved drug screening methods (see Figure 1). Induced pluripotent stem cells (iPSCs) derived from patient skin biopsies can be modified to carry genetic variants associated with neuropsychiatric disorders and differentiated into neurons or glia cells, allowing identification of drugs that correct cellular phenotypes associated with these disorders (see Brain Development).
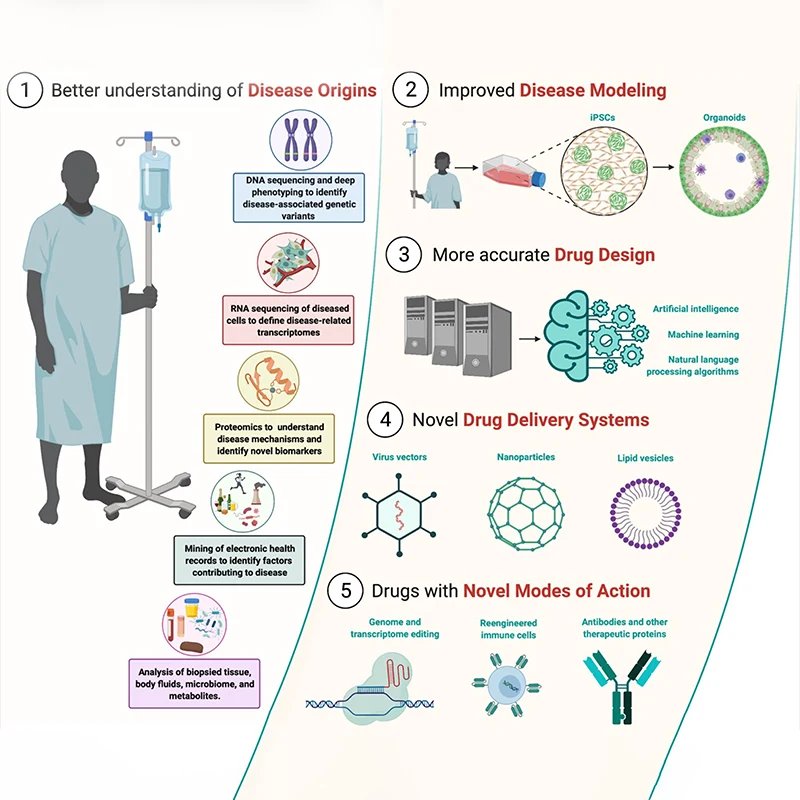
Figure 1. Novel Therapeutics for Brain Disorders
Improvements in genomic and transcriptomic sequencing and proteomics technologies, and artificial intelligence (AI)-based mining of electronic medical records and digital data, will support the identification of new targets for central nervous system (CNS) therapeutic discovery. Advances in AI and other computational approaches will drive more efficient discovery of new drugs that are safe and effective. Cell and tissue engineering will deliver better in vitro models of CNS diseases and facilitate the identification of new drug candidates. New therapeutic modalities will emerge, such as genomic editing to correct genetic variants that contribute to CNS disorders.
Conversely, disease-associated mutations can be corrected in iPSCs-derived brain cells, which can then be delivered to the brains of affected patients. Such stem cell therapies are already in clinical trials for Parkinson's disease, a neurodegenerative disorder caused by the death of movement-related dopamine-containing neurons in the midbrain. High- throughput screening of small molecule libraries to identify new drug candidates is shifting toward more physiologically relevant assay systems, such as iPSC-derived brain cells, 3D brain organoids, and organ-on-a-chip systems. Additionally, fragment-based drug discovery (FBDD), which uses structurally simple and low molecular weight chemical “fragments” that bind to drug targets to assemble complex bioactive compounds, is showing considerable promise.
Over the next decade, artificial intelligence (AI) and machine learning (ML) will play an increasingly important role in advancing our understanding of neuropsychiatric disorders and identifying new treatments. AI and ML algorithms can analyze the enormous amounts of complex data generated by large-scale human genome sequencing experiments, brain imaging studies, tissue biopsies, and information in electronic health records to identify patterns in the data that are otherwise difficult to discern by human researchers (see Figure 1).
AI and ML algorithms can also analyze these complex data to identify new approaches to diagnosis, predict the biological processes that are perturbed in neuropsychiatric disorders, and find innovative targets for drug interventions. As algorithms continue to improve, they will be increasingly used to perform in silico the screening of hundreds of millions of compounds against disease-relevant targets to identify new drug candidates. They will also guide chemistry efforts to improve the potency, selectivity, and safety profile of candidate medications before they enter human clinical trials.
AI and ML algorithms will be used to guide chemistry efforts to improve the potency, selectivity, and safety profile of candidate medications before they enter human clinical trials.
Computer-aided insights into the genetic basis of CNS disorders, combined with technological innovations in genomic sciences, will likely see gene therapy increasingly used to prevent the onset, and delay the progression, of CNS disorders (see Figure 1).
Gene therapy most often involves the use of viral vectors, such as adeno-associated viruses (AAVs), injected either systemically or directly into affected organs of patients to express disease-modifying genetic materials, such as full-length gene sequences, or smaller pieces of genetic material, such as microRNAs to suppress the translation of messenger RNAs (mRNAs) from malfunctioning genes.
For example, voretigene neparvovec is an AAV vector, delivered directly into the eye, that expresses the RPE65 gene and is approved for the treatment of an inherited form of retinal dystrophy. Clinical trials are underway to test a new AAV-based gene therapy called AMT-130 for Huntington's disease (HD), a neurodegenerative disorder caused by a specific mutation in the HTT gene. AMT-130, which is delivered into the striatum of HD patients, expresses a microRNA designed to downregulate expression of this mutant gene.
By leveraging advances in virology, it is conceivable that AAVs can be designed to express their genetic cargos only within specific populations of disease-relevant brain cells, reducing off-target side effects that have plagued traditional small-molecule drugs. Next- generation AAVs that cross the blood-brain barrier may eliminate the need for invasive brain surgeries to deliver gene therapies to the brain.
Nanoparticle-bearing cargos that harness the power of the immune system …
… will likely emerge as novel CNS therapeutics in coming years.
Nanoparticles are emerging as attractive alternatives to virus-based delivery systems for gene therapy. Nanoparticle-mediated delivery of mRNAs encoding the spike protein of SARS-CoV-2 was the basis for the successful COVID-19 vaccines. Nanoparticles that express immune-modulating genetic materials are being developed as new cancer treatments, but the immune system also plays an important role in many CNS disorders, and nanoparticle-bearing cargos that harness the power of the immune system will likely emerge as novel CNS therapeutics in coming years (see Figure 1).
Nanoparticles can be engineered to cross the blood-brain barrier to efficiently transport small-molecule drugs and genetic material to the brain, thereby overcoming a major hurdle for CNS medications development. Nanoparticles are being used to deliver antisense oligonucleotides to the brain, which suppress translation of the targeted RNAs. Antisense oligonucleotide approaches are being piloted for several brain disorders by targeting specific mutated genes that are implicated in these conditions. Organ and cell-type specificity of nanoparticle-based therapies could be achieved by incorporating target sequences for microRNAs that are produced naturally by certain organs and cells into the delivered RNA to inhibit the RNA’s expression where it might produce side effects.
Improved understanding of the genetic basis of CNS disorders, and striking advances in drug development and delivery, promise a new renaissance in the discovery of novel therapeutics for brain disorders.
Over the next decade, the tremendous therapeutic potential of gene- and RNA-editing technologies, such as the CRISPR/Cas9 system and more recently developed iterations, may also be realized. Innovations in genetic and chemical engineering will be required to develop the viruses, nanoparticles, lipid vesicles, modified retrotransposons, and other novel delivery systems necessary to fully harness the power of these technologies. But if this can be accomplished, then the impact will be nothing short of revolutionary, potentially correcting genetic variants that increase risk of CNS disorders before their deleterious actions can be set in motion.
In recent years, most major pharmaceutical companies have scaled back their CNS-focused drug discovery efforts in response to high failure rates in human clinical trials. Improved understanding of the genetic basis and underlying pathophysiology of CNS disorders, and striking technological advances in drug development and drug delivery, promise a new renaissance in the discovery of novel therapeutics for brain disorders.
Featured

Paul J. Kenny, PhD
Ward-Coleman Professor and Chair, Nash Family Department of Neuroscience, and Director, Drug Discovery Institute, Icahn School of Medicine at Mount Sinai

Yizhou Dong, PhD
Professor of Oncological Sciences, Icahn School of Medicine at Mount Sinai