Highlighting the contributions made to the field by Nan Yang, PhD; Deanna Benson, PhD; and Silvia De Rubeis, PhD
Learn how Friedman Brain Institute scientists are exploring the molecular, cellular, and systems-level mechanisms regulating brain development and function and gaining new insights into neurodevelopmental disorders.
Genetic basis of brain function
During the past decade, genetics has been a critical area of focus in understanding autism spectrum disorders (ASDs) and, with the identification of rare and highly penetrant damaging variants and associated genes, much progress has been made. Beyond the immediate diagnostic benefits, these findings have potential for translating discoveries into novel experimental models and drug development for precision treatment.
Genomic advances have revealed that ASD risk genes converge on two key biological pathways: those that encode proteins involved in synaptic function or in chromatin remodeling. Also, gene expression profiling studies have facilitated the identification of the high-risk period for ASDs to the mid-fetal development of the neocortex, specifically in glutamatergic neuronal populations (see Figure 1).
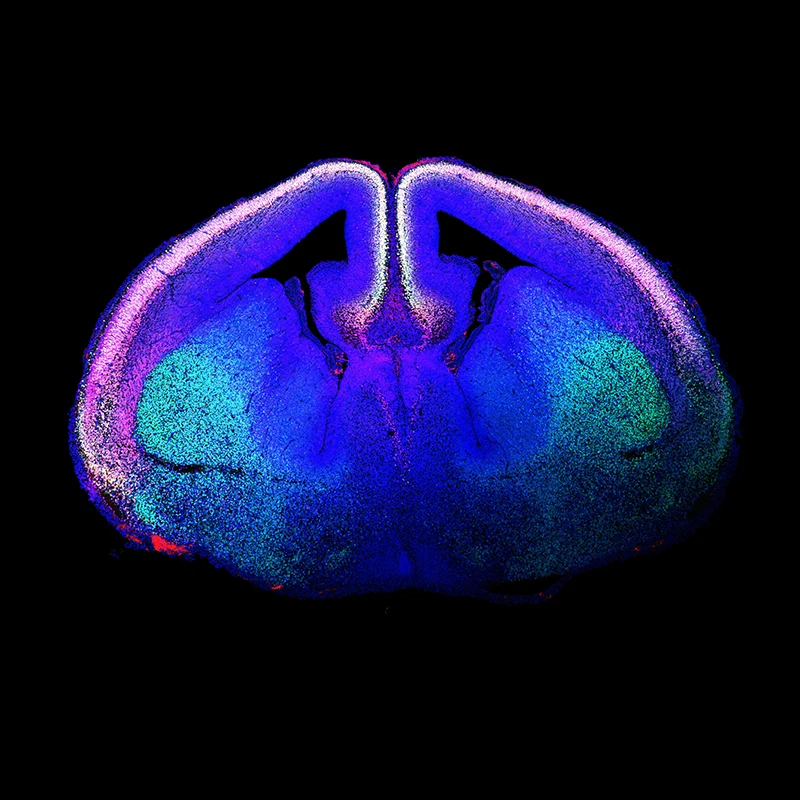
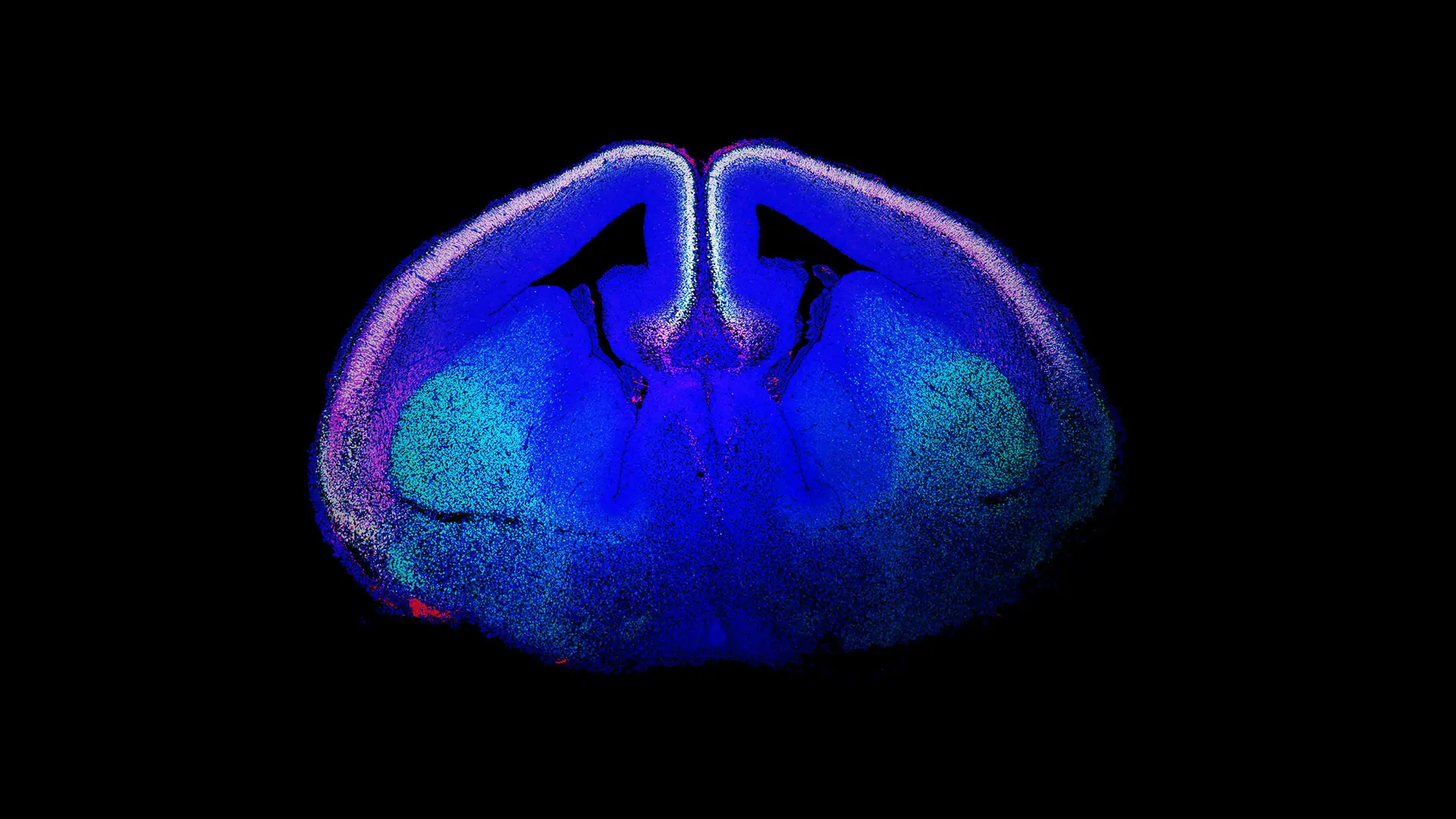
Figure 1. “Rising Life" shows a developing mouse brain, with newborn glutamatergic neurons of the cerebral cortex labeled using immunofluorescence techniques and shown in white. The labeled proteins are CTIP2 (green) and TBR1 (red), with the DNA marker DAPI (blue). White nuclei are glutamatergic neurons that stain for all three. Courtesy of Marta Garcia-Forn, PhD, Postdoctoral Fellow, De Rubeis Lab.
Genetic studies further inform the development of animal models with construct (etiological) validity for ASD-associated mutations. Parallel optogenetic and chemogenetic strategies have unveiled defective circuit functions in these genetic mouse models of ASDs that underlie specific behavioral abnormalities.
For example, these findings have identified neural substrates of normal and disrupted social behavior and their developmental trajectories. Notably, while ASD susceptibility is established during prenatal development, there are many demonstrations in genetic rodent models that correction of the underlying gene abnormality during juvenile periods or even adulthood mitigates disease-related phenotypes. These findings suggest that specific cells and circuits remain malleable and possibly amenable to intervention, offering considerable hope for a new era of therapeutics.
These are just some examples of the pioneering work under way in The Friedman Brain Institute’s Seaver Autism Center for Research and Treatment.
Reconstructing the brain
Over the past 5-10 years, significant progress has been made in developing in vitro human cell models, providing a better understanding of the biology of the human brain and disease etiology. The advent of induced pluripotent stem cells (iPSCs) in 2006 expedited the generation of functional human neural circuits and 3D structures (known as organoids) in a petri dish. The Alper Center for Neural Development and Regeneration of The Friedman Brain Institute organizes numerous efforts at Mount Sinai in this exciting new research area.
Concurrently, genome-editing tools have enabled a relatively high-throughput exploration of genetic variants (including patient-specific mutations) in these in vitro models, which are increasingly yielding significant insights into disease mechanisms for a wide range of neuropsychiatric disorders that include ASDs, schizophrenia, bipolar disorder, and several neurodegenerative disorders. Recent investigations have implanted human iPSC-derived brain organoids into the cortex of newborn rodents and demonstrated the functional integration of the cells within the rodent brain. This approach enables greater developmental maturation of the implanted human cells (which has not yet been possible in vitro) and offers a novel strategy to bridge the gap between human cell models and intact functional circuits that mediate complex behaviors.
Seeing the brain
New imaging technologies have also advanced our understanding of the structure, function, and dynamics of the developing brain. Calcium imaging of fluorescent sensors, coupled with optics that adapt to brain growth, has permitted researchers to examine the emergence of cellular activity and connectivity between brain regions.
In conjunction with other high-resolution methods, such as scanning electron microscopy and spatial transcriptomics, these imaging techniques can assign genetic expression signatures to single cells and cellular domains (see Genetics and Epigenetics). By correlating gene expression data with neuroanatomy and histology, researchers are now identifying novel cell types and circuits, which is improving our understanding of disease pathogenesis and guiding the development of targeted therapies.
These advances in genetics, stem cell biology, and imaging technologies just within the last decade are energizing our scientists as they lead the next wave of discoveries to improve the diagnosis and treatment of neurodevelopmental disorders, and ultimately create better outcomes for patients.
Featured

Nan Yang, PhD
Associate Professor of Neuroscience, Icahn School of Medicine at Mount Sinai

Deanna Benson, PhD
Professor of Neuroscience, Icahn School of Medicine at Mount Sinai

Silvia De Rubeis, PhD
Associate Professor of Psychiatry, Icahn School of Medicine at Mount Sinai