When Filip Swirski, PhD, a leader in cardiovascular immunology research, joined Mount Sinai in 2021 as the inaugural Director of the Cardiovascular Research Institute (CVRI), his stated goal was to build on Mount Sinai’s legacy of discovery and innovation with a renewed focus on lifestyle and prevention, systems physiology and bioengineering, and genetic medicine.
In the ensuing year, CVRI laboratories conducted a broad range of studies that succeeded in advancing the understanding in these areas. These included Dr. Swirski’s study of sleep deprivation and the immune system, a study of myocarditis by Amy Kontorovich, MD, PhD, and the notable publications summarized below.
As it continues to expand, the Institute is hiring faculty, committed to fostering an inclusive and collaborative work environment in which all ideas and identities are valued.
“Scientific discoveries come from the integration of diverse perspectives, discussions, and ideas,” Dr. Swirski says. “And we are committed to nurturing this type of environment.”
Study: Astronauts at Risk for Somatic Mutations
Brojakowska, A., Kour, A., Thel, M.C., Park, E., Bisserier, M., Garikipati, V.N.S., Hadri, L., Mills, P.J., Walsh, K., Goukassian, D.A. Retrospective analysis of somatic mutations and clonal hematopoiesis in astronauts. Commun Biol. 2022 Aug 17;5(1):828. PMID: 35978153.

From right, David A. Goukassian, MD, PhD; Lahouaria Hadri, PhD; and Shihong Zhang, associate researcher.
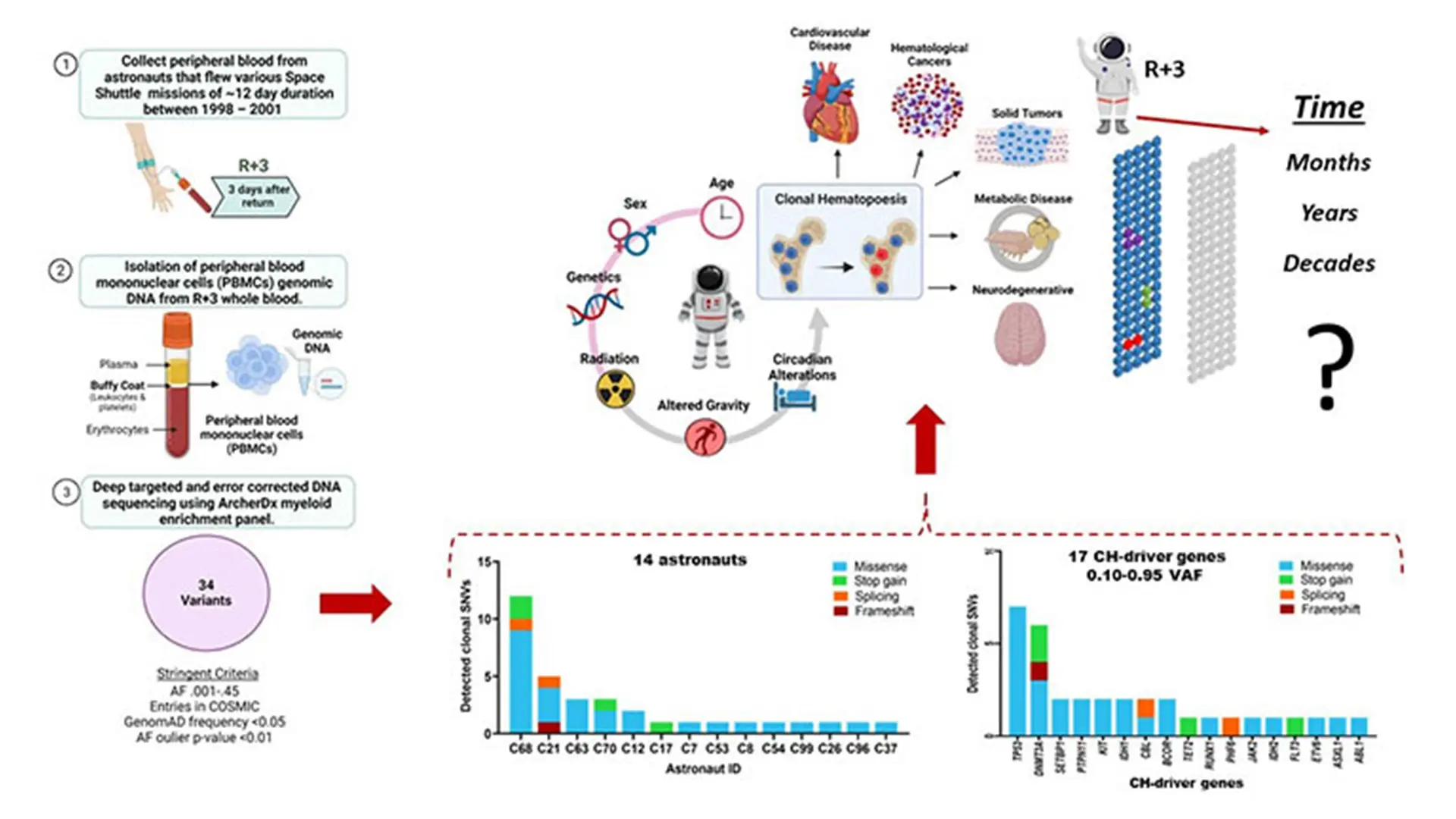
Virtual abstract of the paper "Retrospective analysis of somatic mutations and clonal hematopoiesis in astronauts."
Notes from the Investigator:
David Goukassian, MD, PhD, Professor of Medicine (Cardiology)
Astronauts work in an extreme environment where many factors can result in somatic mutations, most importantly space radiation, which means there is a risk that these mutations could develop into clonal hematopoiesis (CH). There are few signs or symptoms associated with CH; most patients are identified after genetic testing of their blood for other diseases. Although CH is not necessarily an indicator of disease, it is associated with a higher risk for cardiovascular disease, solid and blood cancer.
Given the growing interest in both commercial spaceflights and deep space exploration, we studied, retrospectively, somatic mutation in blood samples from 14 NASA astronauts who flew space shuttle missions between 1998 and 2001. The median age of the astronauts was approximately 42 years old. Approximately 85 percent were male, and 6 of the 14 were on their first mission. We identified 34 mutations in 17 CH-driver genes with at least one mutation in the blood cells in all 14 astronauts studied. The most frequent mutations occurred in TP53, a gene that is mutated in more than 50 percent of all cancers, and DNMT3A, one of the most frequently mutated genes in acute myeloid leukemia. These findings suggest that we can determine the individual susceptibility of astronauts to develop disease related to their work and the importance of early and ongoing screening to assess that susceptibility. Our recommendation is that NASA, and its medical team, screen astronauts for somatic mutations and possible clonal expansion, or regression, every three to five years, and, not less importantly, well into their retirement years when somatic mutations may expand clonally and become a condition known as clonal hematopoiesis of indeterminate potential.
Study: Using modRNA Cocktails to Promote Vascular Regeneration
Kaur, K., Hadas, Y., Kurian, A.A., Zak, M.M., Yoo, J., Mahmood, A., Girard, H., Komargodski, R., Io, T., Santini, M.P., Sultana, N., Sharkar, M.T.K., Magadum, A., Fargnoli, A., Yoon, S., Chepurko, E., Chepurko, V., Eliyahu, E., Pinto, D., Lebeche, D., Kovacic, J.C., Hajjar, R.J., Rafii, S., Zangi, L. Direct reprogramming induces vascular regeneration post muscle ischemic injury. Molecular Therapy. 2021 Oct 6;29(10):3042-3058. PMID: 34332145.

Team members, from left, Magdalena Zak, PhD; Jimeen Yoo, MSc; Lior Zangi, PhD; Gayatri Mainkar, BSc, and Ann Anu Kurian, MSc.
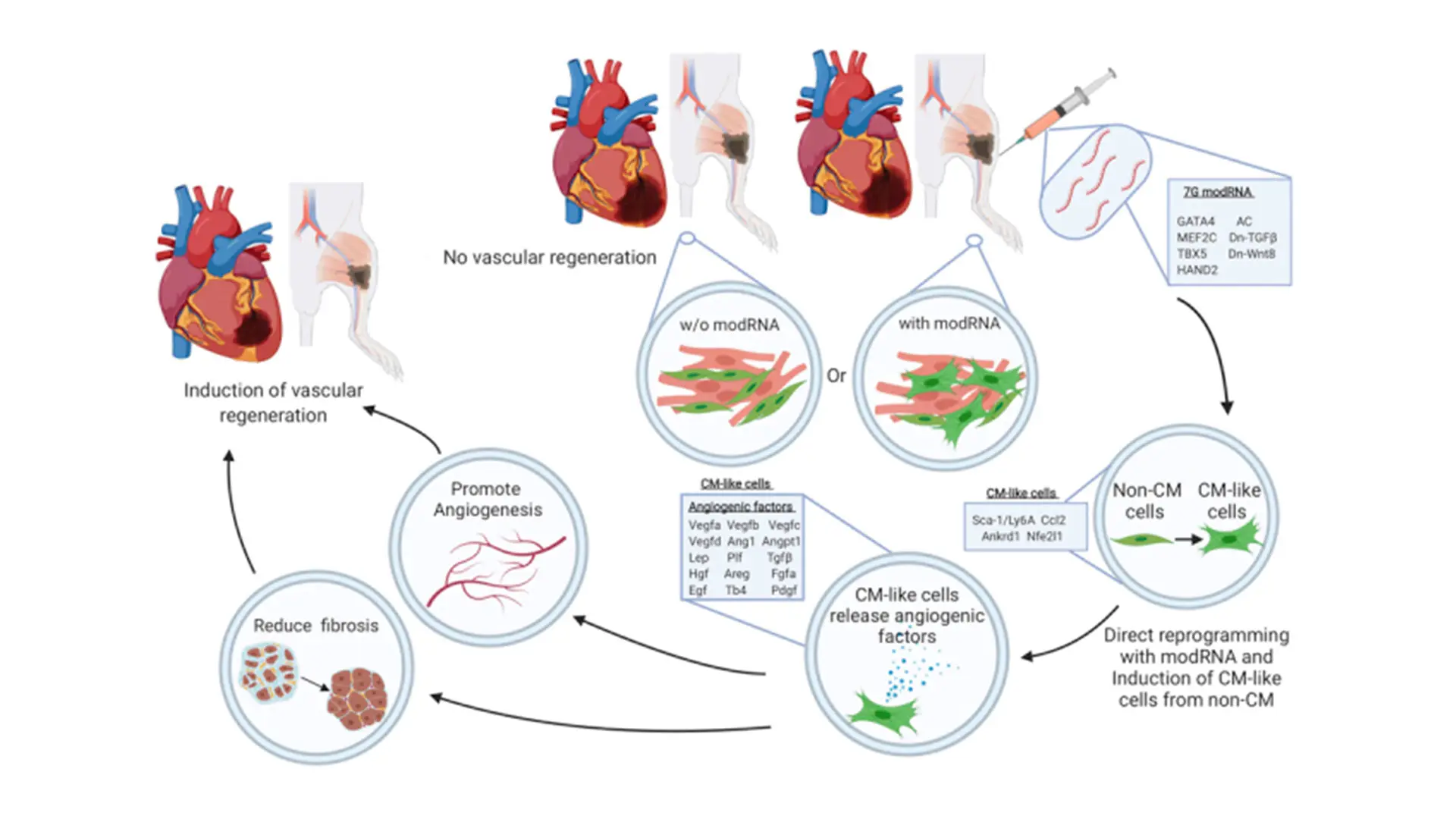
A graphical abstract of the paper "Direct reprogramming induces vascular regeneration post muscle ischemic injury"
Notes from the Investigator:
Lior Zangi, PhD, Associate Professor of Medicine (Cardiology), and Genetics and Genomic Sciences
In this study, we gauge the efficacy of a combinatory modRNA cocktail in direct cardiac reprogramming of non-cardiomyocyte (CM) to CM-like cells. We evaluate the benefits of CM-like cells in the heart and skeletal muscle in vivo. Our study demonstrates efficient, safe, direct reprogramming that promotes vascular regeneration by inducing paracrine angiogenic factors in both human and mouse settings. We show that while 7G-modRNA cannot create significant numbers of de novo, beating CM in vitro or in vivo, it nevertheless led to notably upregulated pro-angiogenic mesenchymeal stromal cells markers and transcription factors. Further, we establish that our 7G-modRNA cocktail leads to neovascularization in ischemic limb injury, indicating that CM-like cells are important in other organs, besides the heart.
For almost 20 years, bone marrow-derived cells have been used in many clinical trials to promote vascular regeneration in patients with acute MI, chronic cardiac ischemia, or chronic critical limb ischemia disease. Years of clinical trials have shown this treatment is safe and leads to modest improvements in physiologic and anatomic parameters, above and beyond conventional therapy. However, the fundamental problems with this method are the poor survival rates of the injected cells and the limited number of ex vivo cultured cells, typically from the same donor. Our approach, by contrast, uses off-the-shelf modRNA cocktails that can convert non-CMs in the scar area to pro-angiogenic mesenchymeal stromal cells (similar to bone marrow-derived cells) without engraftment issues or the need to culture cells ex vivo.
Study: Noninvasive Marker for Pulmonary Hypertension
Maier, A., Liao, S.L., Lescure, T., Robson, P.M., Hirata, N., Sartori, S., Narula, N., Vergani V., Soultanidis, G., Morgenthau, A., Kovacic, J.C., Padilla, M., Narula, J., Jacobi, A., Fayad, Z.A., Trivieri, M.G. Pulmonary Artery 18F-Fluorodeoxyglucose Uptake by PET/CMR as a Marker of Pulmonary Hypertension in Sarcoidosis. JACC Cardiovascular Imaging 2022. 15(1):108-120. PMID: 34274283

Maria Giovanna Trivieri, MD, PhD
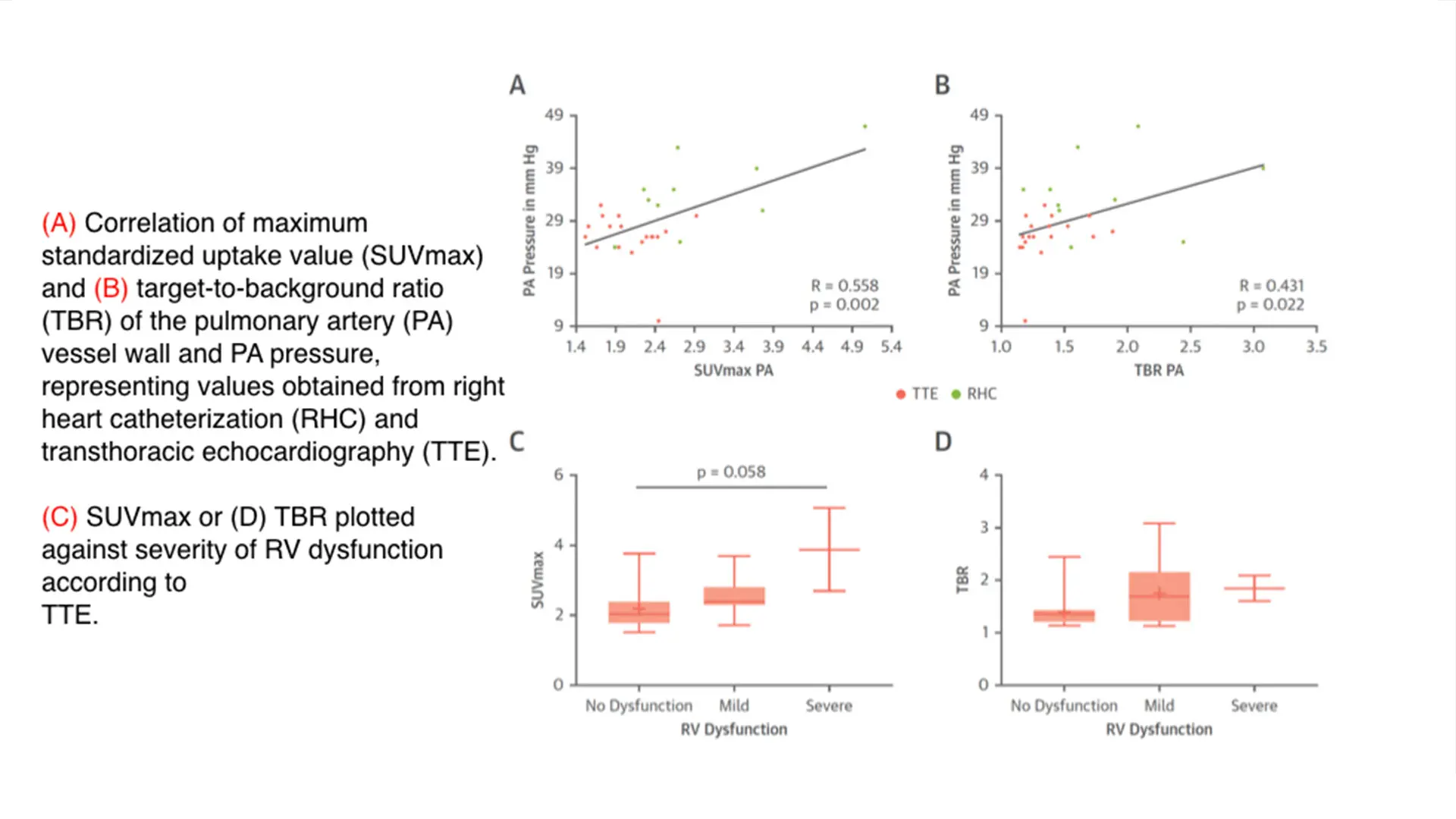
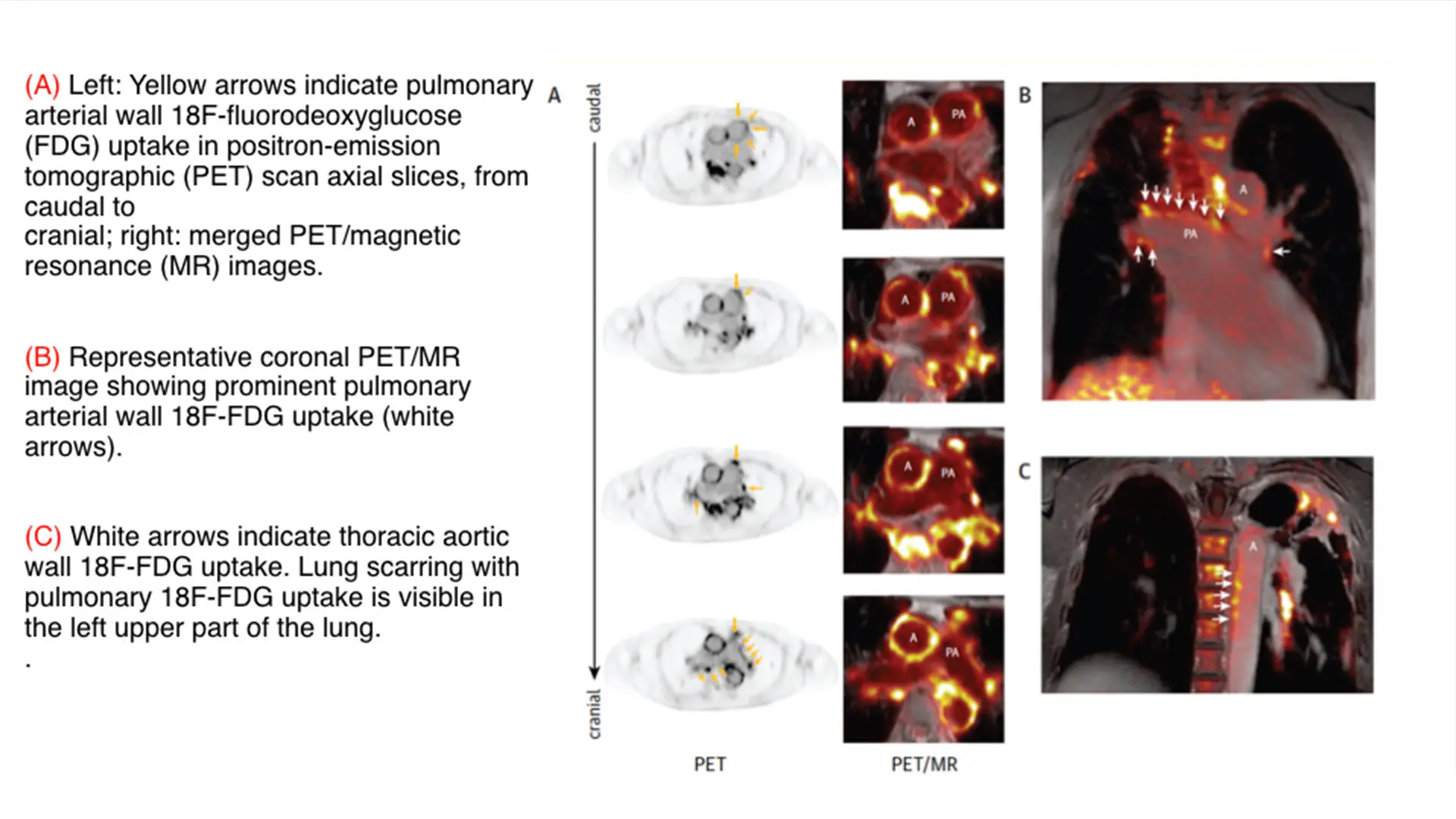
Above and below, figures from the paper "Pulmonary Artery 18F-Fluorodeoxyglucose Uptake by PET/CMR as a Marker of Pulmonary Hypertension in Sarcoidosis"
Notes from the Investigator:
Maria Giovanna Trivieri, MD, PhD, Assistant Professor of Medicine (Cardiology), and Diagnostic, Molecular and Interventional Radiology
Pulmonary hypertension (PH) is a devastating disease, with a very poor prognosis, characterized by narrowing of the artery leading from the heart to the lungs that results in elevation in pressure. If left untreated, this cardiopulmonary condition can lead to failure of the right heart and premature death. Diagnosis of PH presently requires serial catheterization, an invasive procedure. As such, there has been a tremendous effort in seeking alternative tools to replace or integrate this approach.
In our institution, we dispose of cutting-edge imaging modalities that have revolutionized our understanding of a variety of cardiovascular conditions. Particularly promising is 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET), which combined with computed tomography or cardiac magnetic resonance (CMR), offers simultaneous information about “molecular” activity and function or morphology. PET/MR has been used to assess inflammation in the large arteries of the systemic circulation and has provided great insight into the pathophysiology as well as prognosis and treatment response: much less is known about inflammation of the vasculature of the pulmonary system and its relationship to PH.
To test the potential of using this modality in PH, we conducted a single-center cohort study of 175 patients with sarcoidosis, a condition known to be associated to PH. We then identified uptake in the pulmonary artery, which we quantified according to maximum standardized uptake value (SUVmax) and target-to-background ratio (TBR) and compared with available results from right heart catheterization or transthoracic echocardiography. We found that increased pulmonary artery uptake of FDG had good diagnostic accuracy and excellent correlation with the invasive measurements of pulmonary pressure. We concluded that the 18F-FDG uptake in the PA could be used as a noninvasive marker of PH, which we believe has the potential to identify patients with previously undiagnosed PH. Future investigations are planned to evaluate its utility in determining prognosis and monitoring the effects of therapy.
Study: Mechanisms That Relate Aging and Hypertension to Aortic Stiffening
Ma, B., Melton. E., Wiener, R., Zhou, N., Wu, W., Lai, L., Wang, C., Costa. K.D, Qiu, H. Age and Blood Pressure Contribute to Aortic Cell and Tissue Stiffness Through Distinct Mechanisms. Hypertension. 2022;79(8):1777-1788. PMID: 35766034.

Costa Laboratory team members, from left: David Sachs, MS; Kevin Costa, PhD; Helen Orins, PhD candidate; and Robert Wiener, PhD.
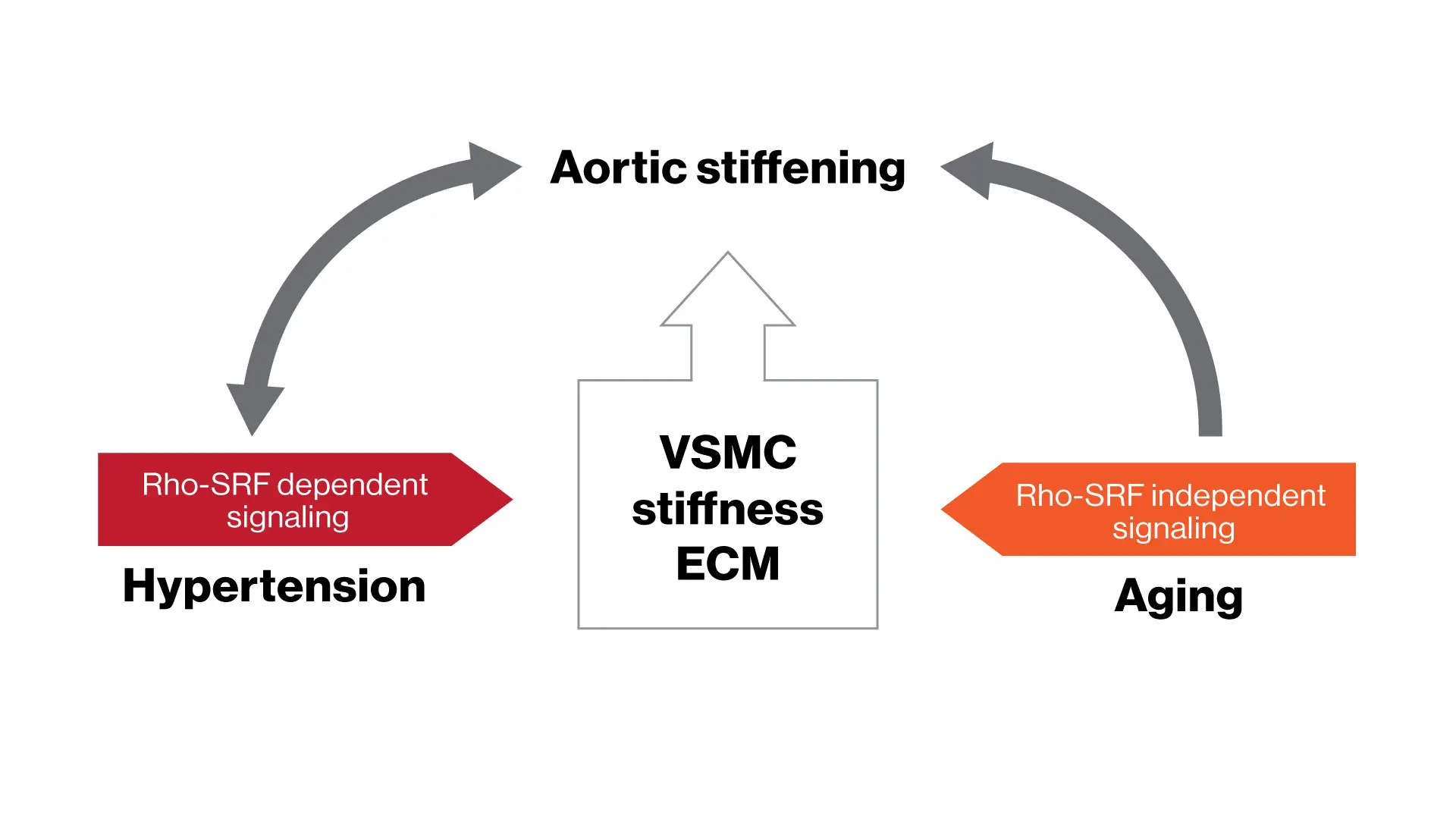
Study results indicate distinct mechanisms mediating aging-associated aortic vascular smooth muscle cell (VSMC) and vessel stiffness, providing new insights into aortic stiffening and the pathogenesis of hypertension in the elderly.
Notes from the Investigator:
Kevin Costa, PhD, Associate Professor of Medicine (Cardiology)
Stiffening of the aorta is strongly associated with both aging and hypertension, but the underlying mechanisms have been unclear. We hypothesized that aging-induced aortic stiffening and hypertension-induced aortic stiffening are mediated by distinct underlying mechanisms. In an ongoing collaboration between the Costa Lab at CVRI and the Qiu Lab at Georgia State University, our research team conducted comprehensive in vivo as well as 2D and 3D in vitro experiments using multiple rodent models to dissect the mechanisms of aortic stiffening mediated by aging and hypertension. We focused on a period representing early-to-middle age, when hypertension and aging begin to affect aortic stiffness without the additional biological complexities of advanced aging.
This study provides novel evidence of distinct mechanisms relating aging and hypertension to the aortic stiffening observed during early-to-middle age, particularly in relation to aortic vascular smooth muscle cell (VSMC) stiffness. Pharmacological inhibition of specific mechano-signaling pathways reduced aortic cell and tissue stiffness associated with hypertension, but did not alter aging-related stiffness. Similarly, particular integrins and VSMC cystoskeletal proteins were specifically associated with hypertension, whereas a shift in filamin isoforms was seen with aging in the absence of hypertension. These new insights suggest that therapeutically counteracting the distinct effects of aging and hypertension may be required for an effective strategy to prevent the development of aortic stiffness in the elderly.
Study: Investigating the Role of the Left Atrium in Heart Failure
Sakata, T., Mazurek, R., Mavropoulos, S.A., Romeo, F.J., Ravichandran, A.J., Watanabe, S., Kariya, T., Ishikawa, K. Impact of ischemia on left atrial remodeling and dysfunction in swine models of mitral regurgitation. American Journal of Physiology-Heart Circulatory Physiology. 2022 Jun 1;322(6):H914-H923. PMID: 35333115

From right: Kiyotake Ishikawa, MD, PhD, with team members Tomoki Sakata, MD; Renata Mazurek, MD; and Jonas Marx, MD, MS.
Kiyotake Ishikawa, MD, PhD
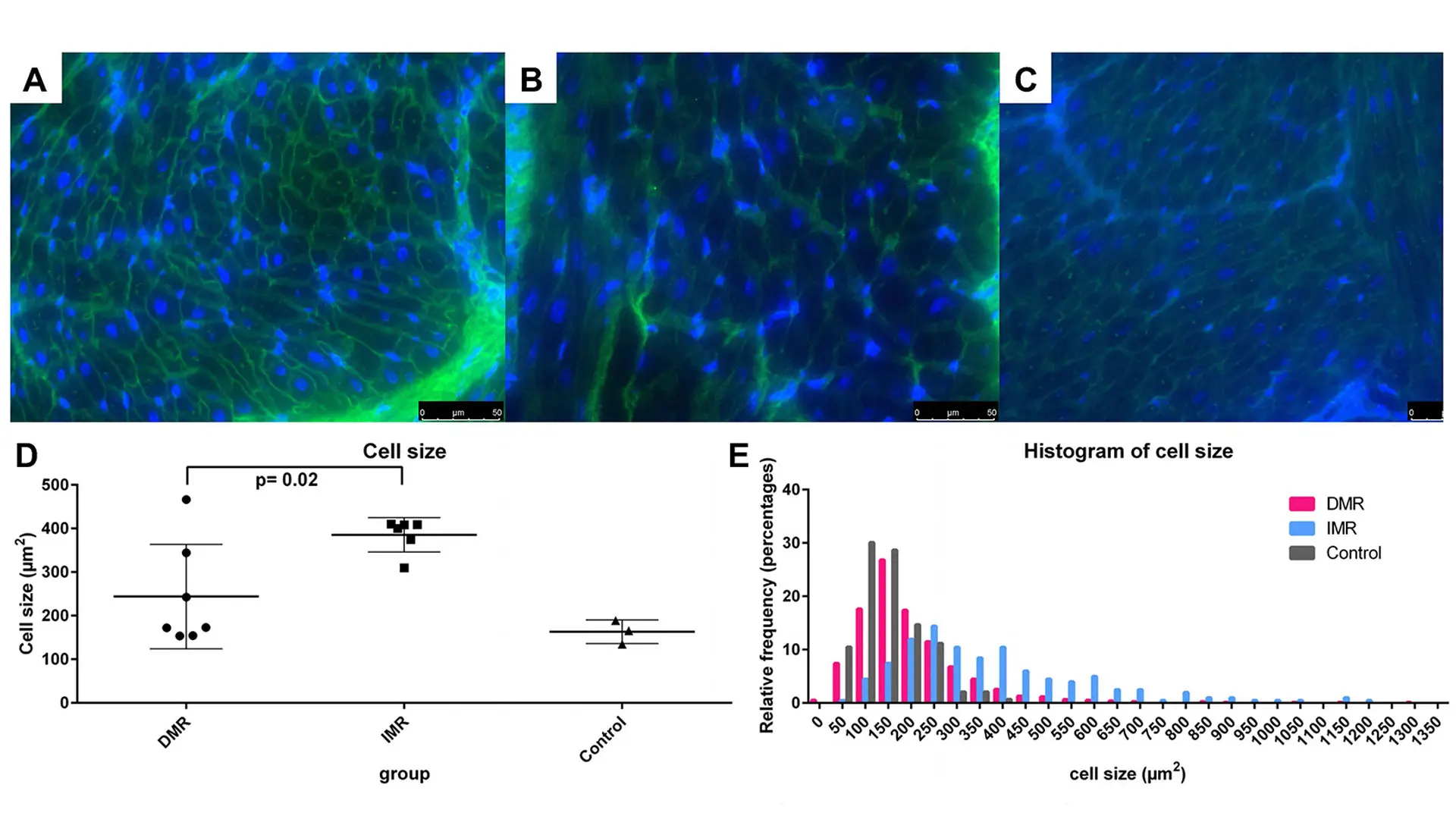
Results of the wheat germ agglutinin staining, in which the size of cardiomyocytes was measured. The degenerative mitral regurgitation group showed similar myocyte size distribution to the sham samples, with a minor increase in the large myocyte fraction.
Notes from the Investigator:
Kiyotake Ishikawa, MD, PhD, Associate Professor of Medicine (Cardiology)
Little is known about how left atrial function and remodeling modulate heart failure. Ischemia is one of the major causes of dysfunction of the left ventricle, and we hypothesized that it also contributes to left atrial pathophysiology. This study demonstrated that left atrial ischemia exaggerates pathological remodeling and promotes atrial dysfunction in a clinically relevant pig chronic heart failure model. Specifically, presence of left atrial ischemia increased the left atrial volume, worsened left atrial emptying function, increased tissue fibrosis and cell size (hypertrophy) compared to the heart failure without left atrial ischemia.
The study prompts further clarification of the role of left atrium in heart failure, and Ishikawa Lab is currently extending this project with model improvement, imaging, and molecular analysis that focuses on fibrosis in the left atrium. Together with a previously established and unique method of in vivo closed-chest left atrial pressure-volume relationship assessment, we expect subsequent studies will improve hemodynamic, physiological, and molecular understanding of left atrial ischemia and its contribution to chronic heart failure.
Study: Defining a Pathway of Atherosclerotic Disease
Leece, L., Xu, Y., V’Gangula, B., Chandel, N., Pothula, V., Caudrillier, A., Santini, M.P., d’Escamard, V., Ceholski, D., Gorski, P.A., Ma, L., Koplev, S., Bjørklund, M.M., Björkegren, J.L., Boehm, M., Bentzon, J.F., Fuster, V., Kim, H.W., Weintraub, N.L., Baker, A.H., Bernstein, E., Kovacic, J.C. Histone deacetylase 9 promotes endothelial-mesenchymal transition and an unfavorable atherosclerotic plaque phenotype. Journal of Clinical Investigation. 2021 Aug 2;131(15):e131178. PMID: 34338228

Jason Kovacic, MD, PhD
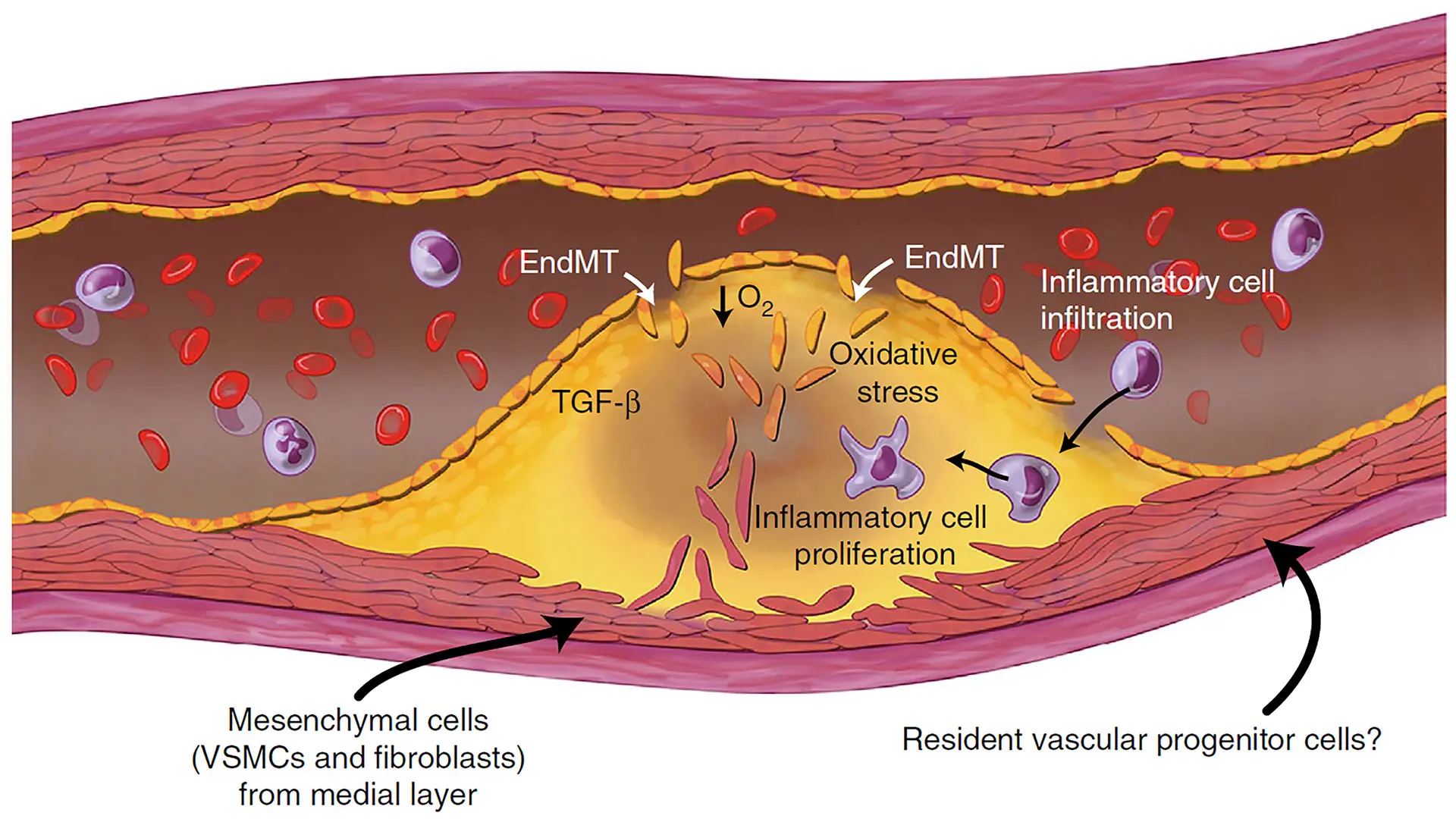
A schematic illustration of the cellular contributions to atherosclerotic plaques, where EndMT (endothelial to mesenchymal transition) plays a key role. (From a related 2016 paper, "Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability" in Nature Communications.)
Notes from the Investigator:
Jason Kovacic, MD, PhD, Professor of Medicine (Cardiology)
Endothelial-mesenchymal transition (EndMT) is associated with various cardiovascular diseases and in particular with atherosclerosis and plaque instability. However, the molecular pathways that govern EndMT are poorly defined. Specifically, the role of epigenetic factors and histone deacetylases (HDACs) in controlling EndMT and the atherosclerotic plaque phenotype remains unclear. Here, we identified histone deacetylation, specifically that mediated by HDAC9 (a class IIa HDAC), as playing an important role in both EndMT and atherosclerosis.
Using in vitro models, we found that class IIa HDAC inhibition sustained the expression of endothelial proteins and mitigated the increase in mesenchymal proteins, effectively blocking EndMT. Similarly, ex vivo genetic knockout of HDAC9 in endothelial cells prevented EndMT and preserved a more endothelial-like phenotype. In vivo, atherosclerosis-prone mice with endothelial-specific HDAC9 knockout showed reduced EndMT and a significantly reduced plaque area. Furthermore, these mice displayed a more favorable plaque phenotype, with reduced plaque lipid content and increased fibrous cap thickness. Together, these findings indicate that HDAC9 contributes to vascular pathology by promoting EndMT. Our study provides evidence for a pathological link among EndMT, HDAC9, and atherosclerosis and suggests that targeting of HDAC9 may be beneficial for plaque stabilization or slowing the progression of atherosclerotic disease.
