Chronic, insufficient sleep can negatively affect immune cells, which may lead to inflammatory disorders and cardiovascular disease, according to a study led by Filip Swirski, PhD, Director of the Cardiovascular Research Institute at the Icahn School of Medicine at Mount Sinai. More specifically, consistently losing an hour and a half of sleep a night potentially increases the risk.
The research, published in September 2022 in the Journal of Experimental Medicine, is the first to show that sleep interruption restructures the epigenome of hematopoietic stem and progenitor cells (HSPCs) and increases their proliferation, thus reducing hematopoietic clonal diversity through accelerated genetic drift. Sleep fragmentation exerts a lasting influence on the HSPC epigenome, skewing commitment toward a myeloid fate and priming cells for exaggerated inflammatory bursts. Immune cells fight infection, but if the number of these cells gets too high, they overreact and cause inflammation. The study is also the first to show that catching up on sleep doesn’t reverse the effects of sleep disruption.
“This study begins to identify the biological mechanisms that link sleep and immunological health over the long term. It shows that in humans and mice, disrupted sleep has a profound influence on the programming of immune cells and rate of their production, causing them to lose their protective effects and actually make infections worse—and these changes are long-lasting,” says lead author Dr. Swirski.
Combining hematopoietic clonal tracking with mathematical modeling, the team inferred that sleep preserves clonal diversity by limiting neutral drift. In humans, sleep restriction alters the HSPC epigenome and activates hematopoiesis. These findings show that sleep slows decay of the hematopoietic system by calibrating the hematopoietic epigenome, constraining inflammatory output, and maintaining clonal diversity.
“This is yet another key observation that sleep reduces inflammation and, conversely, that sleep interruption increases inflammation,” Dr. Swirski says. “This work emphasizes the importance of adults consistently sleeping seven to eight hours a day to help prevent inflammation and disease, especially for those with underlying medical conditions.”
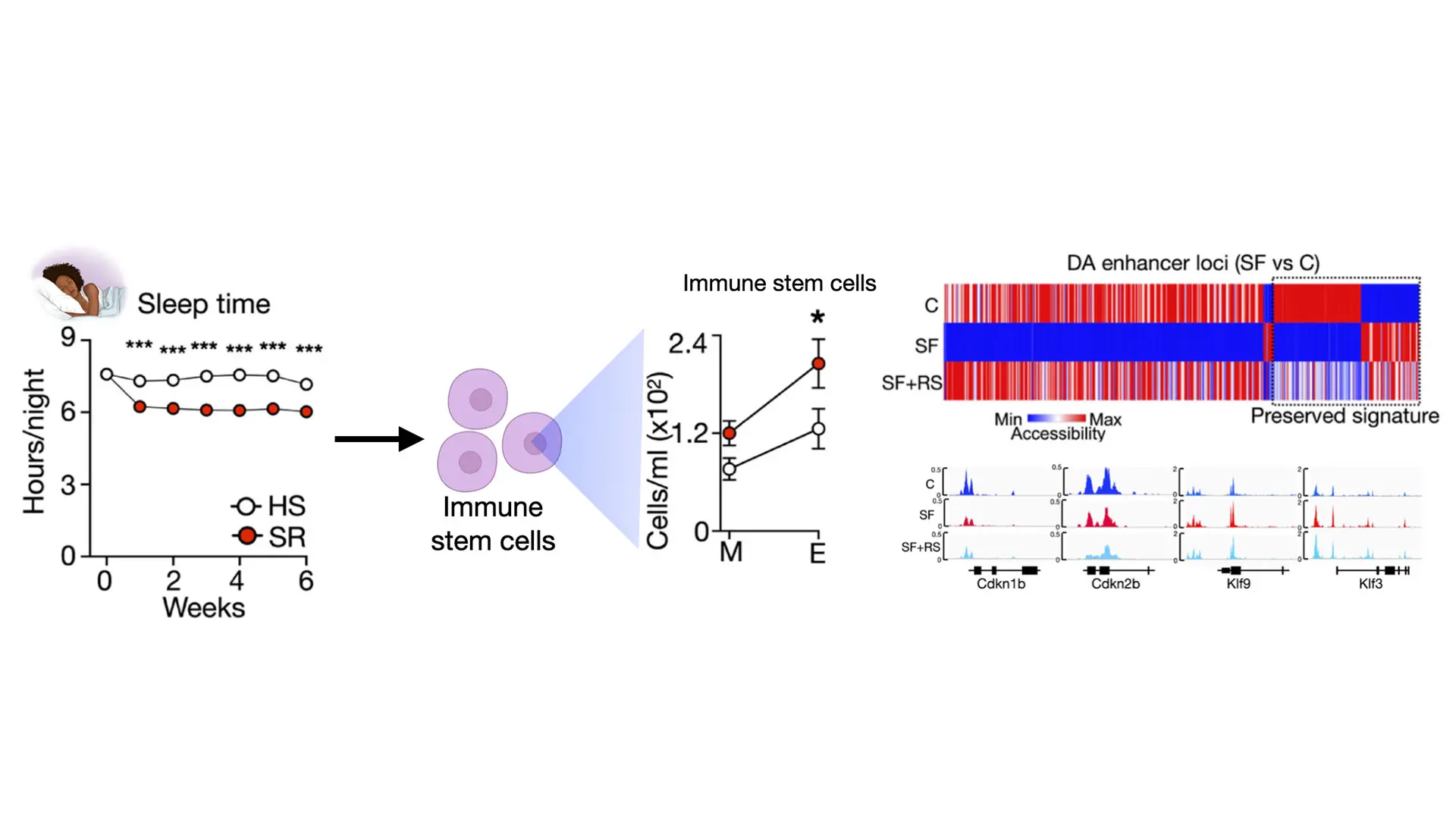
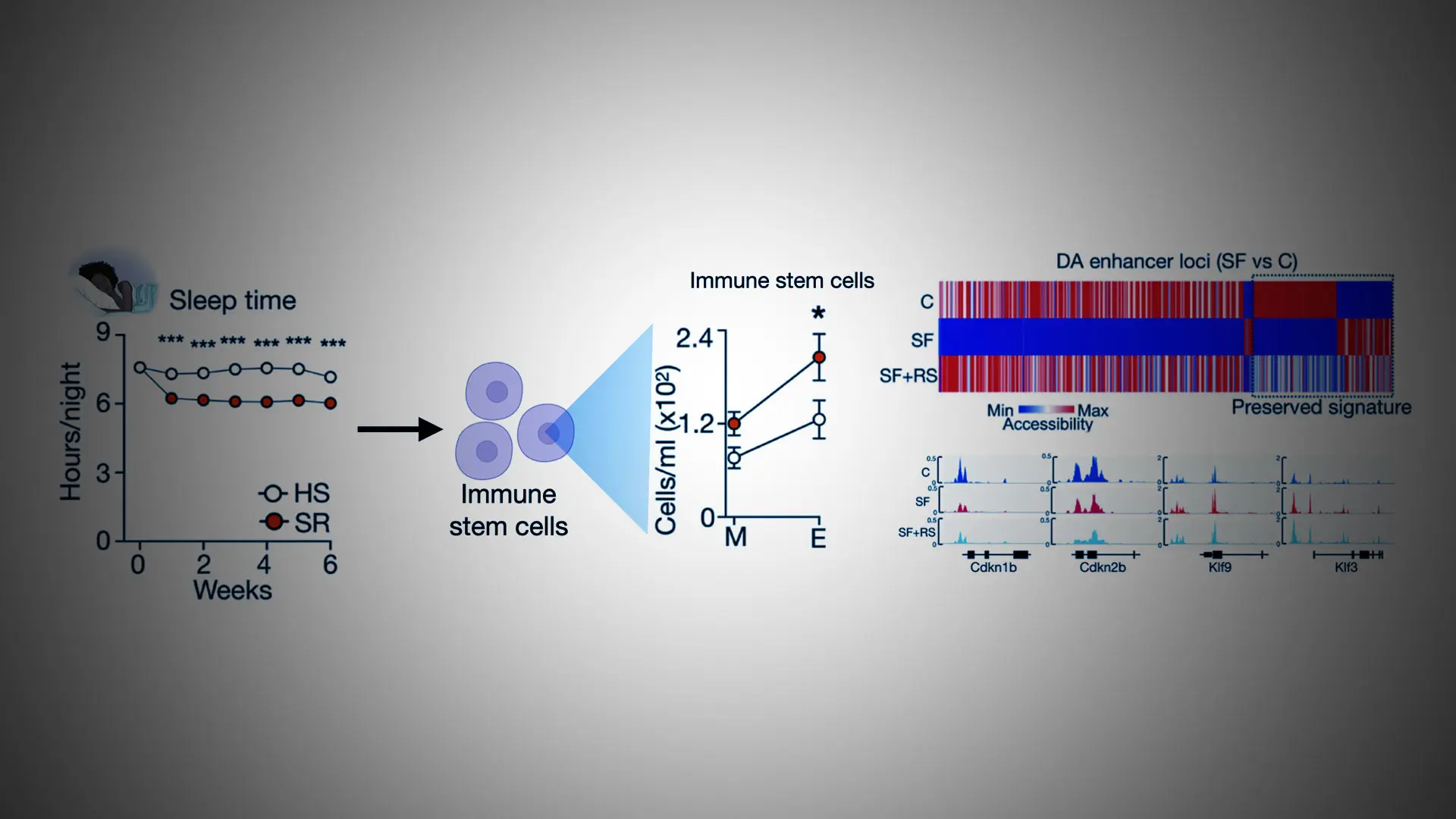
The left side of the figure shows the sleep time of the humans in the study. Participants in the sleep restriction group (the red dots) slept less over the six weeks. The right side of the figure shows the level of stem cells and locations in the genome that were rewired.
A team of investigators analyzed 14 healthy adults who regularly sleep eight hours a night. First, researchers monitored them sleeping at least eight hours a night for six weeks. They drew their blood and analyzed their immune cells. Then, the same group of adults reduced their sleep time by 90 minutes every night for six weeks, and had their blood and immune cells reanalyzed. At the end of the study, researchers compared the blood and cell samples from the full night’s sleep and restricted sleep periods. All participants had significant changes in their immune cells (also known as hematopoietic cells) due to a lack of sleep—there were more of them, and the DNA structure was altered. After six weeks of sleep restriction, they had an increased number of immune cells.
Researchers also analyzed sleep in mouse models. Groups of mice were either allowed to sleep undisturbed, or had sleep fragmentation, where they were awakened throughout the night for 16 weeks. Then, mice with sleep fragmentation went through uninterrupted sleep recovery for ten weeks. Investigators took immune stem cells and immune cells from mice during these undisturbed, fragmented, and sleep recovery phases, analyzed them, and compared them at the end of the experiment.
Results in mice were consistent with results in humans. They showed that all mice with fragmented sleep had significant changes to their immune stem cells, producing an increased number of immune cells, and also showed evidence of rewiring and reprogramming. A notable finding from the mouse group was that even after sleep recovery, the immune stem cells retained this rewiring structure, and they continued to produce additional white blood cells, making the mice susceptible to inflammation and disease.
“Our findings suggest that sleep recovery is not able to fully reverse the effects of poor-quality sleep. We can detect a molecular imprint of insufficient sleep in immune stem cells, even after weeks of recovery sleep. This molecular imprint can cause the cells to respond in inappropriate ways, leading to inflammation and disease,” says co-lead investigator Cameron McAlpine, PhD, Assistant Professor of Medicine (Cardiology) at Icahn Mount Sinai. “It was surprising to find that not all clusters of stem cells responded to insufficient sleep in the same way. There were some stem cell clusters that proliferated and grew in number, while other clusters became smaller. This reduction in overall diversity and aging of the immune stem cell population is an important contributor to inflammatory diseases and cardiovascular disease.”
The National Heart, Lung, and Blood Institute; and the National Center for Advancing Translational Sciences, part of the National Institutes of Health, helped fund this study.
Featured

Filip K. Swirski, PhD
Director of the Cardiovascular Research Institute, Arthur and Janet C. Ross Professor of Medicine (Cardiology), and Professor of Diagnostic, Molecular, and Interventional Radiology

Cameron McAlpine, PhD
Assistant Professor of Medicine (Cardiology), and Neuroscience