In 2024, Mount Sinai launched the Center of Excellence for Gynecologic Cancer, dedicated to providing a multidisciplinary approach to diagnosing and treating gynecologic cancers, and integrating leading-edge medical science with compassionate patient care. The Center also prioritizes cancer prevention with a focus on genetic predisposition to cancers, as well as preinvasive conditions that affect women's health. The Center of Excellence is part of The Tisch Cancer Institute, a National Cancer Institute-designated cancer center, and offers a comprehensive range of services, including advanced gynecologic cancer screening and diagnosis, supportive care, and a wide range of clinical trials.
The Center is led by co-Directors Stephanie V. Blank, MD, Director of Gynecologic Oncology for the Mount Sinai Health System, Associate Director of The Tisch Cancer Institute, Women’s Cancers, and Professor of Obstetrics, Gynecology and Reproductive Science, Icahn School of Medicine at Mount Sinai; and Dmitriy Zamarin, MD, PhD, Section Head of Gynecologic Medical Oncology, Mount Sinai Health System, and Professor of Hematology and Medical Oncology, Icahn School of Medicine at Mount Sinai.
“The launch of the Center of Excellence for Gynecologic Cancer represents a crucial step forward in our commitment to addressing the evolving needs of our diverse community,” Dr. Blank says. “We want to make sure that cancer patients can live the best life possible. It is not only about their treatment; we’re talking about nutrition, fertility, psychological support, and genetics. And because it’s all in one place, we can make it easily accessible to our patients.”
Dr. Blank points out the site reached the status of Center of Excellence because of its multidisciplinary approach, exceptional expertise, a highly respected fellowship, and a truly patient-centered approach to care. The Center maintains a robust research portfolio, including investigator, industry, and National Institutes of Health-sponsored clinical trials and preclinical research programs that seek to advance the field and offer the latest, most promising therapies and diagnostic and preventive options to patients.
Mount Sinai’s Center of Excellence is equipped to diagnose and treat all forms of gynecologic cancers, including cervical, fallopian tube, ovarian, uterine, vaginal, and other cancers, including those of the peritoneum and vulva. Physicians in the Center include leading gynecologic oncologists, medical oncologists, physician-scientists, clinical trialists, radiation oncologists, gynecologic pathologists, radiologists, and reproductive endocrinologists. There is expertise in radical surgery and minimally invasive surgery via both laparoscopic and robotic platforms, as well as fertility-sparing surgery. The Center offers genetic counseling and testing and survivorship follow-up, including cancer surveillance, menopause management, sexual health, oncofertility, and nutrition.
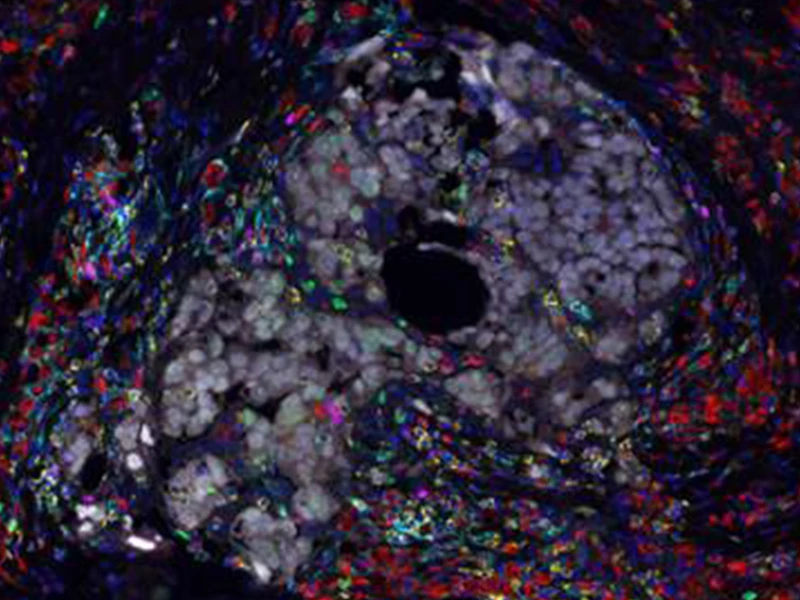
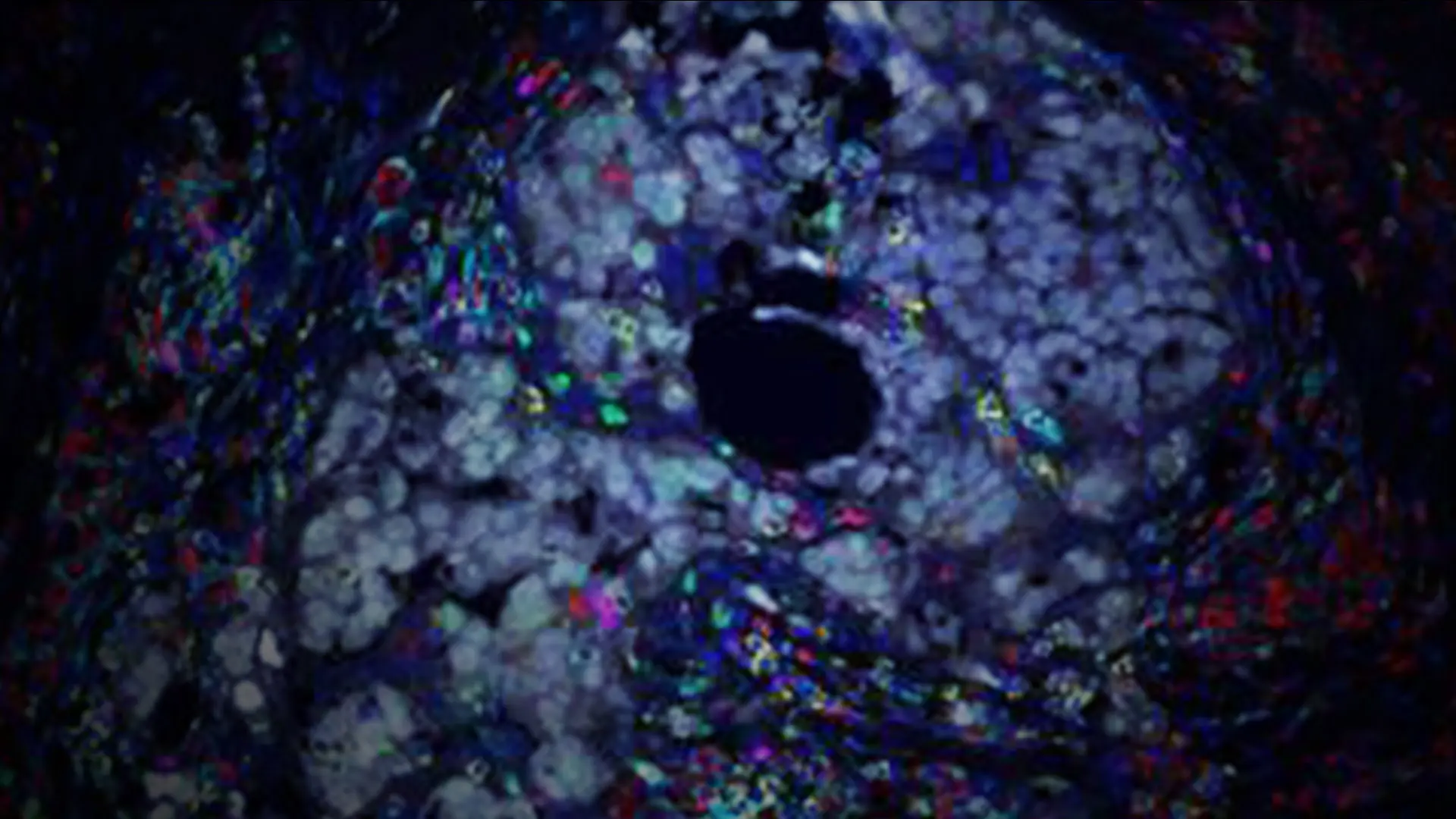
Multiplex immunofluorescence image of ovarian tumor isolated from a patient treated with chemotherapy and immunotherapy. Image demonstrates cancer cells (gray) surrounded by immune cells including macrophages (red) and T cells (yellow).
Clinical Trials Are Advancing the Field
Clinical trials at the Center of Excellence are exploring a range of advanced therapies. These include targeted therapies, small molecule drugs that specifically target and kill cancer cells based on specific genetic alterations such as mutations in these cells. Another novel class of drugs called antibody-drug conjugates (ADCs) is a highly targeted approach in which molecules are designed to deliver a toxic payload into cancer cells by exploiting molecular targets on cells’ surfaces. The Center is also exploring cancer immunotherapies—therapeutic strategies designed to unleash the power of the immune system against cancer. One such novel approach exploits the drugs called bispecific T-cell engagers—immunotherapy drugs that bring immune cells directly to the site of tumors, helping them recognize and kill cancer cells.
The Center is also conducting trials designed to optimize the detection of tumors at debulking surgery for ovarian cancer, and preventive trials with the potential to establish a new standard of care in risk-reducing surgery for those at increased genetic risk for ovarian cancer. Clinical trials now underway include:
A randomized, phase 1b study to assess the safety, tolerability, pharmacokinetics, and efficacy of sovilnesib at different dose levels to establish the recommended phase 2 dose in subjects with high-grade serous ovarian cancer.
An open-label, phase 1 study seeking to determine the optimal dose of sacituzumab govitecan, an ADC-targeting trophoblast cell surface antigen 2 (TROP2), for use in combination with cisplatin for treatment of platinum-sensitive epithelial ovarian and endometrial cancers.
A phase 2 trial testing an ADC known as disitamab vedotin, which targets tumors expressing human epidermal growth factor receptor 2 (HER2). The trial is testing the drug in patients with previously treated ovarian and endometrial cancers that have recurred.
A phase 3, randomized, controlled study to compare the efficacy and safety of MK-2870, another ADC that targets TROP2, to standard treatment in participants with endometrial cancer whose cancer has recurred.
A randomized, phase 3 study of mirvetuximab soravtansine, an ADC that targets the folate receptor, in combination with bevacizumab versus bevacizumab alone as maintenance therapy for patients with platinum-sensitive recurrent ovarian cancers that express this receptor.
A study to evaluate the safety and tolerability of XmAb®541, a bispecific T-cell engager targeting claudin-6, in advanced cancers.
A phase 3 study of Gleolan™ (aminolevulinic acid hydrochloride) to enhance visualization of tumors in patients with suspected, newly diagnosed, or recurrent ovarian cancer. This study, opening at the end of January, uses a dye to make sure all of the cancer is visible at ovarian cancer debulkings.
WISP2: A study of risk-reducing salpingectomy (removal of the tubes) with delayed oophorectomy (removal of the ovaries) as an alternative to risk-reducing salpingo-oophorectomy (removing tubes and ovaries) in high-risk women to assess the safety of prevention.
SOROCk: A nonrandomized prospective clinical trial comparing the noninferiority of salpingectomy to reducing salpingo-oophorectomy to reduce the risk of ovarian cancer among people who have BRCA1 mutations.
“This new center stands at the forefront of gynecologic cancer care, reflecting our dedication to advancing treatment while also meeting the changing needs of our community,” Dr. Zamarin says. “By leveraging leading-edge research and addressing current health trends, we are positioned to offer innovative, effective solutions for gynecologic cancers with sensitivity to our diverse patient population. Our goal is to enhance both the quality of care and the quality of life for our patients, ensuring that our services are both responsive and proactive with respect to emerging challenges in women's health.”
The Mount Sinai Hospital is ranked No. 8 in the nation for cancer by U.S. News & World Report for 2024-25. Additionally, The Mount Sinai Hospital is rated “High Performing” for Gynecological Cancer Surgery by U.S. News & World Report.
For more information about the Mount Sinai Center of Excellence for Gynecologic Cancers, please visit www.mountsinai.org/gynonc.
Featured

Stephanie V. Blank, MD
Director of Gynecologic Oncology, Professor of Obstetrics, Gynecology, and Reproductive Science

Dmitriy Zamarin, MD, PhD
Head of Gynecologic Medical Oncology, Tisch Cancer Institute, Professor of Medicine, Hematology, and Medical Oncology