Biopsy of the brain is also impossible as a matter of routine because removal of even a rice-grain size of tissue may, depending on its location, cause irreversible impairment in crucial brain functions. This lack of access to the pathological tissue uniquely for the brain dramatically impedes our ability to study the origins of many neurological and psychiatric illnesses.
Mount Sinai is one of six National Institutes of Health-sponsored biorepositories for procuring, storing, and distributing post-mortem brain tissue for study.
This core challenge in neuroscience underscores the importance of studying brain tissue postmortem, which requires the collection of tissues in what are often referred to as “brain banks.” Mount Sinai has long invested in a brain collection and is one of six sites that form the National Institutes of Health (NIH) sponsored NeuroBioBank. Together, these sites, which adhere to the strictest ethical standards when acquiring specimens, serve as a national resource for scientific discovery. Mount Sinai’s brain bank collection has historically been one of the largest in the country under the longtime leadership of Vahram (Harry) Haroutunian, PhD.
Recently, neuropathologist and neuroscientist John F. Crary, MD, PhD, was recruited to provide long-term vision to the brain-banking program at Mount Sinai with a charge to deploy innovative technology that would enable scientists to overcome the barriers that limit access to—and the characterization of—the tissues that the bank collects. Today, Mount Sinai uses an open sharing model and specimen bar coding system, which together expands access to these very valuable specimens, increases the speed in which tissues are shared, decreases costs, and accelerates the pace of discovery.
Still, most brain banks use relatively antiquated techniques that have changed little in the past 100 years. For example, most still manually and painstakingly examine each case with a microscope to help determine a diagnosis, an approach that is laborious, slow, expensive, and biased.
Mount Sinai is also using artificial intelligence (AI) to allow researchers to address complex data modalities, neuroanatomy, and clinical symptomatology. This is in collaboration with the Windreich Department of Artificial Intelligence and Human Health at the Icahn School of Medicine at Mount Sinai. These AI approaches are extremely good at identifying patterns in large brain datasets that conventional methods could never accomplish, all enabled by the deployment of
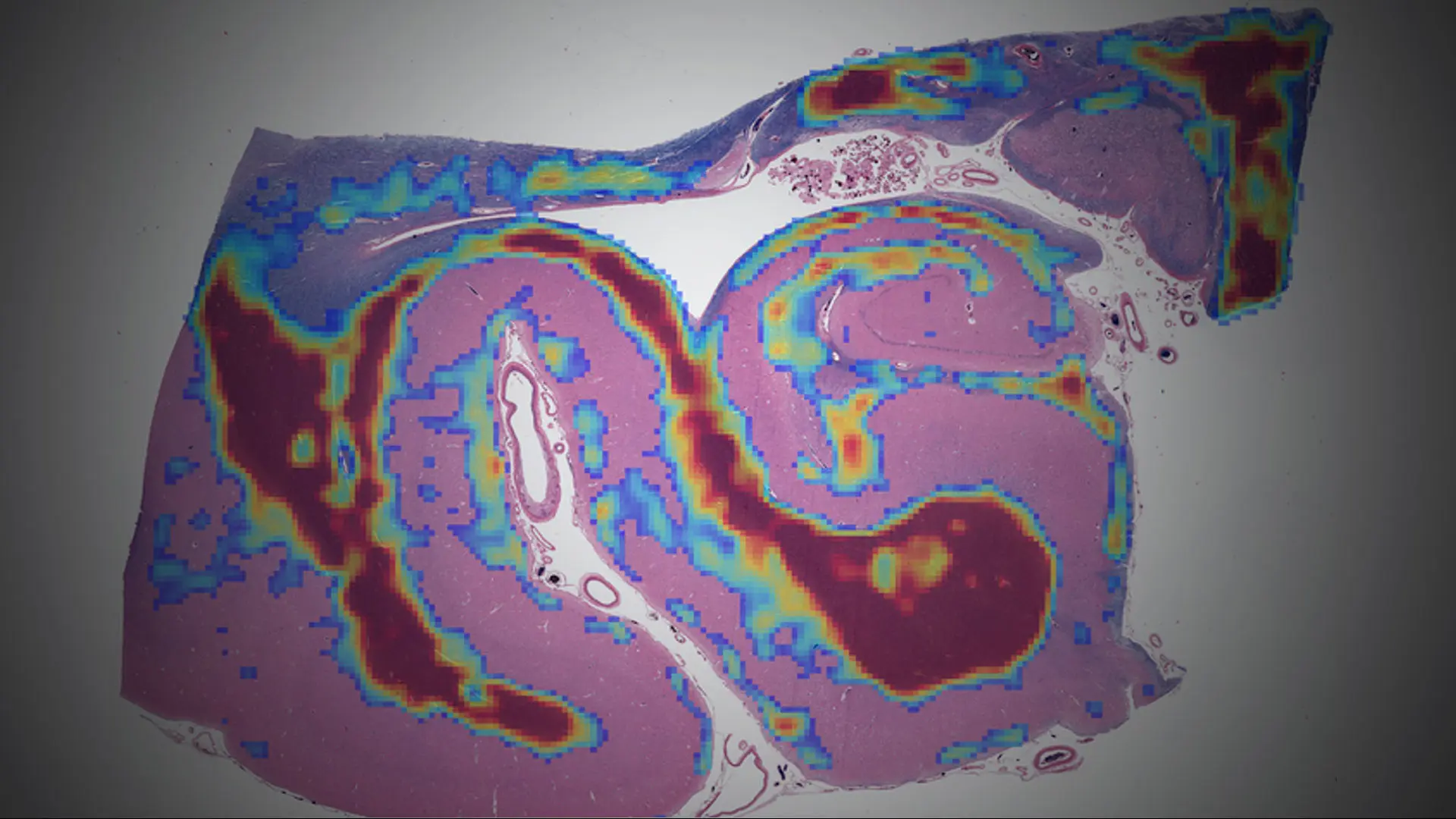
high-throughput whole-slide image scanners that allow the creation of large, microscopic brain image datasets that can be analyzed on Mount Sinai’s high-performance computing resource known as Minerva (See Figure 1).
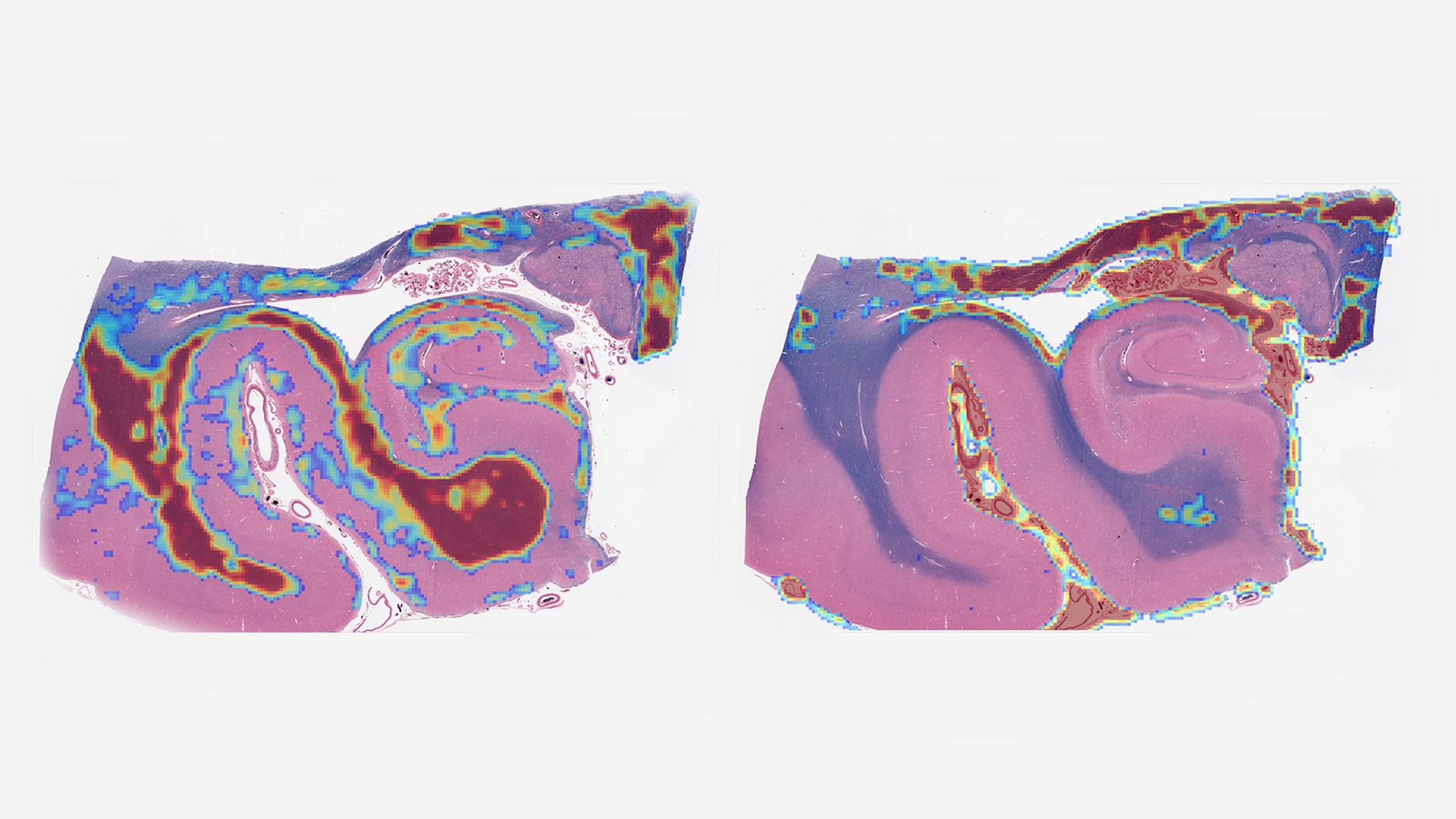
Figure 1. Digital whole-slide images of a human hippocampus analyzed using an age-acceleration algorithm developed by Gabriel Marx, MD, a research track Neurology resident at Mount Sinai. The Rainwater Charitable Foundation is funding Mount Sinai investigators to use AI to measure histological features too complex or subtle for human observers.
The Neuropathology of Alzheimer’s Disease and the Related Dementias
Dr. Alois Alzheimer was among the first neuropathologists to define changes that occur in the brains of people with dementia. His analysis of postmortem brains from people who died with dementia defined what we now refer to as Alzheimer’s disease, a condition characterized by two neuropathological abnormalities that he first described in these postmortem studies in the early 1900s: amyloid plaques and neurofibrillary tangles.
However, in more recent years, it has become clear that Alzheimer’s disease is actually one of many different disease states with different neuropathological features and presumably different etiologies and pathophysiological mechanisms. Just as cancer is a collection of afflictions, each requiring personalized treatments, many scientists now believe that dementia must be subdivided into numerous entities and that a singular focus on amyloid is short-sighted.
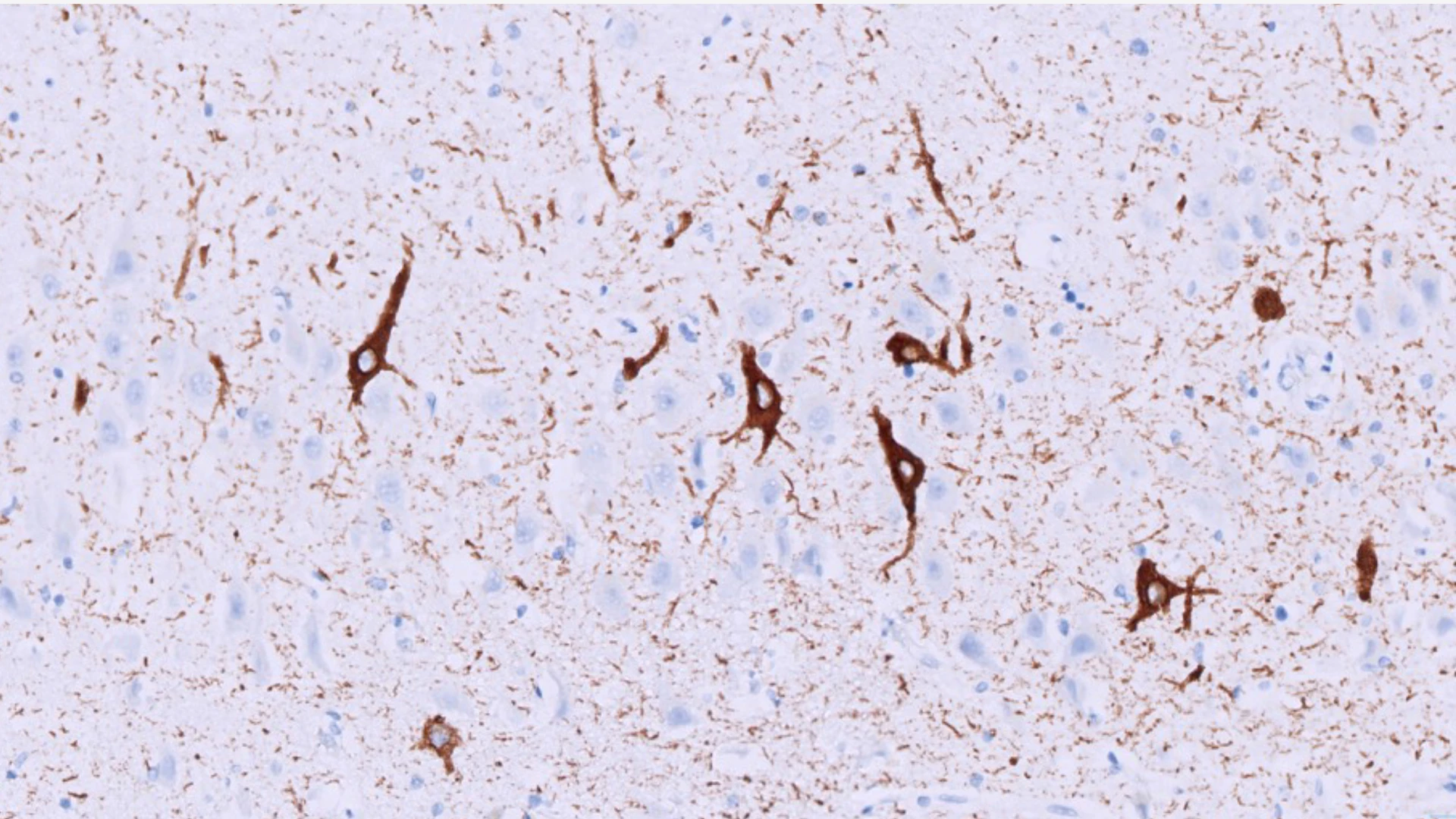
Figure 2: Microscopic image of neurons with tangles (stained in brown) in a patient with primary age-related tauopathy (PART). John Crary, MD, PhD, discovered that these patients who lack amyloid plaques are often misdiagnosed with early Alzheimer’s disease. Brain banks are critical for discovering what causes memory impairment so that precision therapeutics can be developed.
Dr. Crary has focused his research on what he refers to as “Alzheimer’s mimics,” forms of dementia that are misdiagnosed as Alzheimer’s disease but do not meet the diagnostic criteria established originally by Dr. Alzheimer at autopsy.
Using this approach, Dr. Crary published a groundbreaking paper in 2014 describing a new disease named primary age-related tauopathy (PART) that explains impairment in up to 7 percent of dementia patients (See Figure 2). Dr. Crary sees enormous potential in continuing to identify individual differences among dementia patients and expects these insights will pave the way toward more precise diagnostics and individualized treatments.
New Focus on Movement Disorders and Traumatic Brain Injury, and More
Historically, Mount Sinai’s brain collection had focused on Alzheimer’s disease and psychiatric disorders, such as schizophrenia. Dr. Crary soon realized, however, that the Mount Sinai research community increasingly required postmortem samples from patients with other diseases—especially movement disorders, such as Parkinson’s disease, and those with traumatic brain injury (TBI).
The brain bank requires referrals from clinicians who have engaged closely with patients and their families over the course of illness. “Most are enthusiastic about meaningfully contributing to science by providing the gift of knowledge and hope,” says Dr. Crary. Initially, new brain specimens were procured slowly, but grew in number during the COVID-19 pandemic, when the potential for neurological involvement in COVID-19 became a reality. In 2020, the program banked nearly 100 brains from patients who had died from their COVID-19 illnesses.
Within its first five years, the brain bank has contributed to more than 1,000 research projects as it continues to expand its mission. Major collaborations include a study on Parkinson’s disease in veterans with TBI with Ruth Walker, MD, PhD, and a national effort to create a single-cell 3D map of an entire human brain with Patrick Hof, MD (see Figure 3).
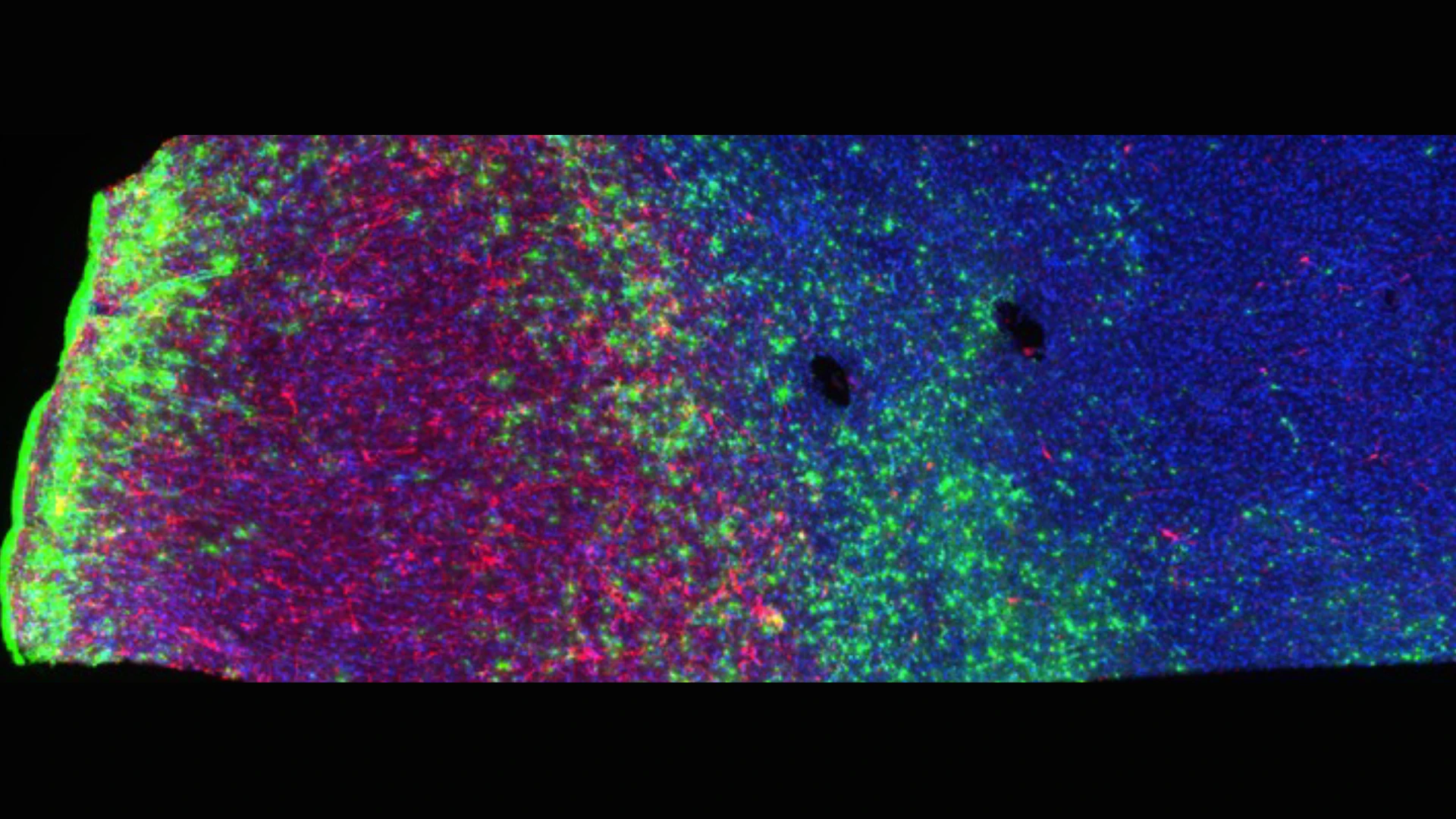
Figure 3. Stained human brain tissue showing neurons (red), astrocytes (green), and microglia (blue). This NIH-funded project is an ambitious attempt, for the first time, to map all the cells in an entire human brain.
The brain bank has also supported ongoing projects by Towfique Raj, PhD, on the contribution of brain inflammation in Parkinson’s disease; Kurt Farrell, PhD, on the largest genomic study of frontotemporal dementia supported by CurePSP; Fanny Elahi, MD, PhD, on developing tests for cerebrovascular disease; and Kristen Dams-O’Connor, PhD, on characterizing different forms of TBI, including intimate partner violence, as described in this related story, along with many others. Recently, Jamie M. Walker, MD, PhD, who is doing pioneering studies of brain aging using spatial proteomics, joined the growing brain bank team as co-director.
Brain bank efforts also include a robust commitment to train the next generation of neuropathologists. Dr. Crary, for example, conducts a brain-cutting session that draws large audiences interested in this growing subspecialty. Additionally, the brain bank hosts case-review conferences on rare and complex diseases with neurologists and neurology residents. Mount Sinai volunteer and intern programs have also attracted eager participants, some of whom have been inspired to attend medical school to specialize in neuropathology. Other programs recruit college interns from underserved communities to teach them about the importance and promise of neuropathology.

The Mount Sinai brain bank is a nexus for teaching. John Crary, MD, PhD, left, hosts sessions with trainees who are interested in a career in translational brain science.
“The potential of brain banks is immense,” says Dr. Crary, as he acknowledges the funding support Icahn Mount Sinai has received for new initiatives that has come from a number of organizations, including the Rainwater Charitable Foundation, CurePSP, Neuroacanthocytosis Advocacy USA Foundation, and the Mac Parkman Foundation. One emerging collaboration is the ambitious NeuroAI Foundation’s “10,000 Brains Project” that aims to create a vast digital brain bank to comprehend the human brain across populations.
“Patients with neurologic conditions and their caregivers are always on my mind,” says Dr. Crary. “I deeply admire their enormous strength. Giving them hope is my goal. I want them to know we are working as hard and as fast as we can on their behalf.”
Featured

John F. Crary, MD, PhD
Director of the Mount Sinai Neuropathology Brain Bank and Research CoRE, and Professor of Pathology, Molecular and Cell-Based Medicine, and Neuroscience

Vahram (Harry) Haroutunion, PhD
Professor of Psychiatry, and Neuroscience, and Associate Director for Research for the Veterans Administration Mental Illness Research and Education Clinical Center

Gabriel Marx, MD, MSBDS
Research Track, Neurology Resident

Jamie M. Walker, MD, PhD
Co-Director, Mount Sinai Neuropathology Bank Bank and Research CoRE, and Associate Professor of Pathology