Today, research aims to better understand the pathological basis of TBI based on its many different etiologies, course of injury (from acute to chronic), and severity (from mild to severe), with the ultimate goals of developing and evaluating improved diagnostic tests as well as more effective treatments for this highly heterogeneous syndrome. A related goal is to identify and understand the many factors that influence the long-term course of TBI to optimize outcomes for individual patients.
Read on to learn how the research portfolio of the BIRC is growing, with a focus on three areas: efforts to understand the brain pathology associated with intimate partner violence (IPV), which is at epidemic proportions, yet grossly under recognized; the use of machine learning to better classify different symptomatic subtypes of TBI; and new approaches to enhance care for patients living with TBI.
Uncovering the Neuropathological Signatures of the Silent Epidemic: Intimate Partner Violence
It is estimated that one in three women experiences IPV in their lifetime; incredibly, one in four has experienced IPV by the age of 24. Perpetrators commonly target the head where visible wounds may be obscured by a woman’s hair, making TBI a common and often invisible consequence of IPV.
The clinical and neuropathological signatures of repetitive head impacts, such as those sustained in contact sports, have been extensively investigated in the past 15 years since chronic traumatic encephalopathy (CTE) was diagnosed in the brain of a retired National Football League player. CTE is believed to result from repeated head trauma, causing a progressive brain syndrome that results in clinical dementia.
However, CTE can only be definitively diagnosed after death by studying postmortem brain tissue for the pathognomonic patterns of phosphorylated tau deposition. (Phosphorylated tau in other brain regions is also a pathognomonic feature of Alzheimer’s disease and frontotemporal dementias.) The number of women living with IPV globally dwarfs the number of elite male contact sport athletes, and yet autopsy reports of IPV-related brain injury are nearly nonexistent.
The patterns and contexts of brain injury sustained by IPV survivors are distinct from those sustained by contact sport athletes in important ways. Physical violence sustained over months or years may result in multiple moderate-to severe TBIs, repeated subclinical head impacts, and anoxic/hypoxic injury consequent to suffocation or nonfatal strangulation. This wide range of potential injuries associated with IPV prompted Mount Sinai researchers to question the assumption that CTE is the primary pathological consequence of IPV-related brain trauma.
The number of women living with IPV globally dwarfs the number of elite male contact sport athletes, and yet autopsy reports of IPV-related brain injury are nearly nonexistent.
— Kristen Dams-O’Connor, PhD
Recently, investigators at the BIRC, directed by Kristen Dams-O’Connor, PhD, forged a collaboration with the New York City Office of the Chief Medical Examiner, which enabled unprecedented investigation into the neuropathology of IPV.
The team applied the network-based autopsy protocol developed for the Late Effects of TBI (LETBI) study, which features high-resolution ex vivo neuroimaging to guide tissue sampling and ensure comprehensive lesion characterization.
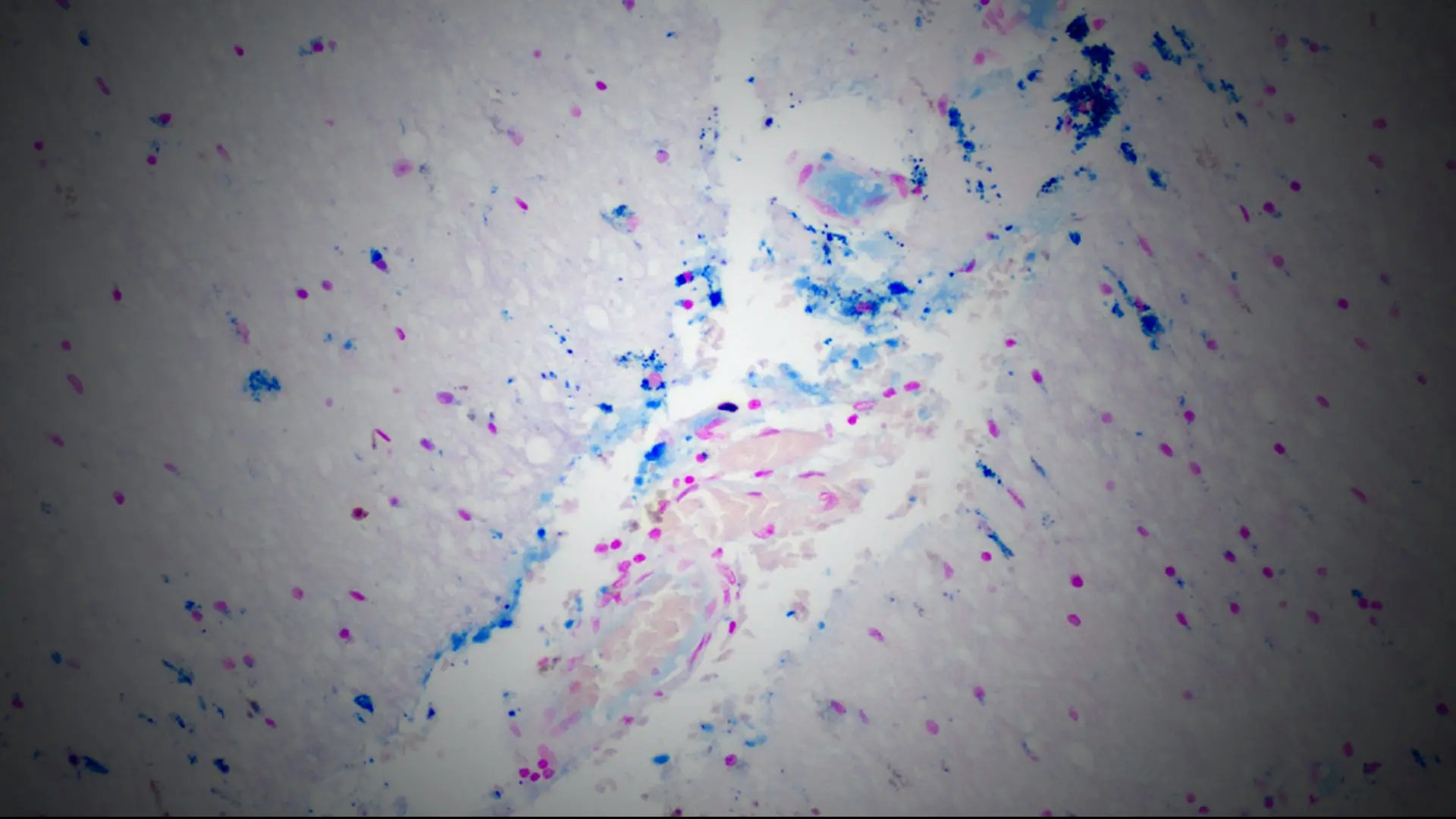
In 14 prospectively collected cases (median age = 4th decade), the researchers found evidence of remote and acute TBI, nonfatal strangulation, and a dramatic burden of cerebrovascular, neurological, and psychiatric disease. Ex vivo magnetic resonance imaging (MRI) identified subtle vascular pathology, which was sampled for characterization of perivascular rarefaction and edema indicative of compromised vessel integrity and accumulation of proteinaceous fluid suggestive of blood-brain barrier breakdown. Diffuse axonal injury was evident, alongside perivascular and parenchymal iron deposition and microgliosis (see Figure 1).
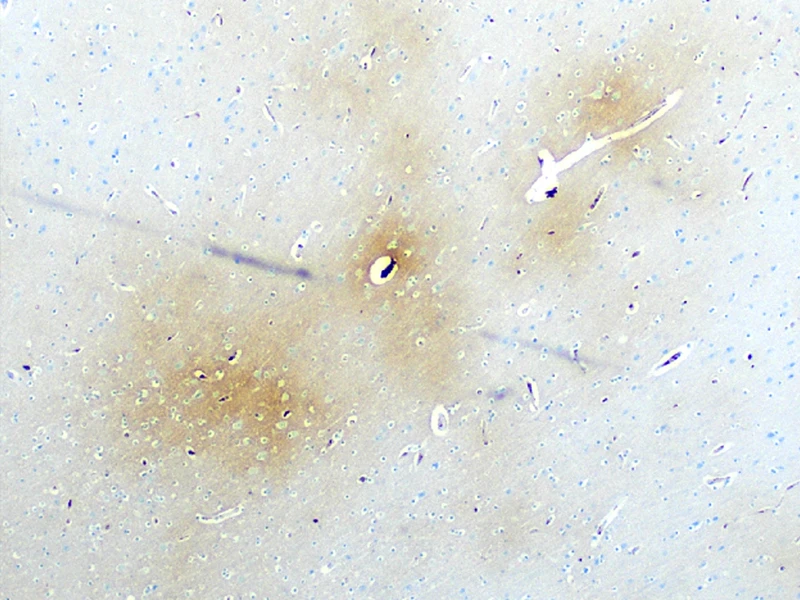
Figure 1. Fibrinogen around microvessels (brown staining on left) in the frontal white matter indicating acute vascular injury; iron deposition (blue staining on right) in right thalamus with subthalamic nucleus marking the site of remote trauma-induced red blood cell breakdown.
Lacunae and/or chronic infarcts were evident in six cases. Alzheimer’s disease-associated neuropathologic changes were detectable in only a single case: the oldest in the series. No CTE neuropathologic changes were found in any case. The team replicated these findings with an international network of collaborators, permitting exploration in 70 cases with a history of IPV. Vascular and white matter pathologies were evident in this archival series, but neurodegenerative disease was rare (median age = 4th decade). No cases met consensus criteria for CTE neuropathologic changes.
The pathology documented in this study, which represents the largest autopsy case series of IPV in the world, likely reflects the cumulative contribution of multi-etiology brain injuries, life course trauma, and comorbid and poorly managed medical and psychiatric conditions, each of which has implications for brain health and dementia risk. The findings provide early evidence that vascular disease may play an important role in clinical symptomatology and progressive decline in some survivors of IPV-related brain injury: a finding that encourages hope to the extent that it can be identified and treated.
The pathology documented in our study represents ...
... the largest autopsy case series of intimate partner violence in the world.
While cause of death was undetermined for several cases in the prospective New York City series at the time of publication, cause of death was overdose or homicide in at least half of the women. IPV is a silent epidemic that surged during the COVID-19 pandemic, the consequences of which society has not yet begun to understand. Many survivors of IPV indicate they would have disclosed their abuse had they been asked. Rigorous screening using structured trauma-informed methods can save lives. This underscores the importance of proper training for doctors, nurses, and other health care professionals.
The BIRC at Mount Sinai is dedicated to continuing to study the psychological and neurobiological sequelae of IPV-related brain injury. One example is a large multicenter National Institutes of Health-funded study led by Carrie Esopenko, PhD. A better understanding of IPV will improve treatment while focusing society’s attention on this all too often ignored crisis.
Using Machine Learning to Classify Distinct Neurobehavioral Phenotypes After TBI
While TBI researchers have long opined that “heterogeneity” is a hallmark of TBI and its clinical outcomes, the empirical data to support this dogma is still in its nascency. Many studies, including the field’s prominent Phase 3 clinical trials, utilize global outcome measures based on gross categories that are sometimes dichotomized further into “good” or “poor” recovery. The field has relied for decades on a three-tiered system to classify injury severity: “mild,” “moderate,” and “severe.” Limited understanding of heterogeneous clinical phenotypes of chronic TBI has impeded trial selection, treatment specificity, and availability of evidence-based interventions for TBI.
Using rich multimodal clinical data from the NIH-funded LETBI study, the BIRC team is pioneering a novel phenotyping method for chronic TBI. The LETBI study collects a comprehensive multimodal battery of clinical tests to measure cognitive, motor, psychological, and behavioral function, and motor skills, as well as structural/ functional brain MRI scans.
Using rich multimodal clinical data, Mount Sinai scientists are ...
... pioneering a novel phenotyping method for chronic TBI.
With expertise spanning biostatistics, clinical neuropsychology, neurology, radiology, and neuropathology, the team makes use of the large amounts of multimodal data available.
BIRC faculty member Raj G. Kumar, PhD, MPH, led analyses designed to classify distinct neurobehavioral phenotypes after TBI through advanced methods in artificial intelligence and machine learning.
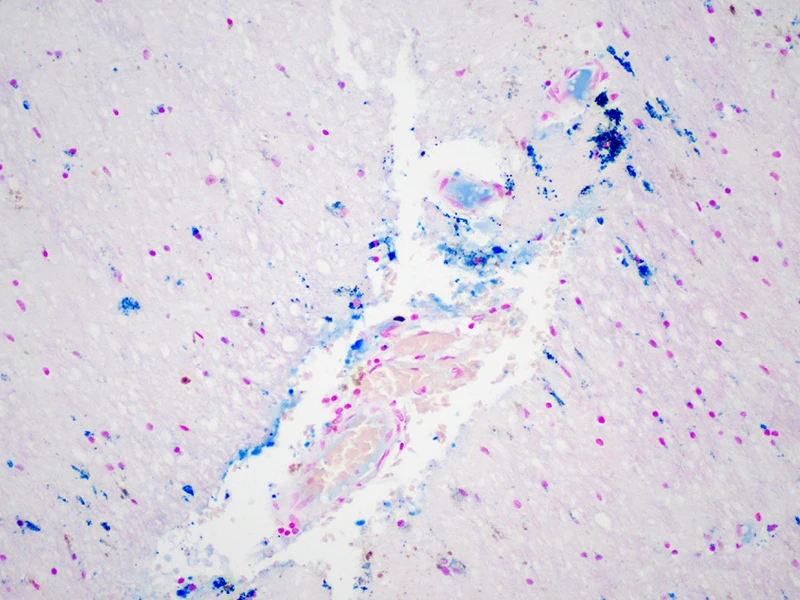
Figure 2. Using machine learning to classify TBI phenotypes.
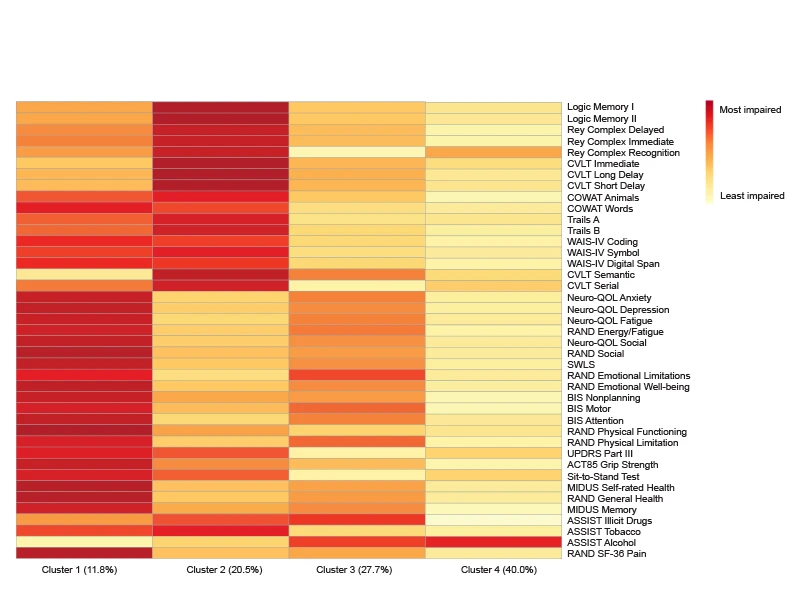
Figure 3. Heat map characterizing average neurobehavioral measures by cluster assignment.
As depicted in Figures 2 and 3, we documented four distinct “clusters” that represent different phenotypes of persons living with TBI. The clusters qualitatively represent: Cluster 1: Global Deficits; Cluster 2: Predominant Cognitive Deficits; Cluster 3: Predominant Mood and Behavioral Deficits; and Cluster 4: Relative Favorable Recovery. We used contemporaneous brain MRI data to identify the neuroanatomical signatures of each of these four phenotypes.
Using a network-based approach corresponding to each of the seven neural networks in the canonical Yeo atlas, we found that individuals in the phenotype characterized by Predominant Cognitive Deficits (Cluster 2) display lower average brain volumes in executive control, dorsal attention, default mode, and visual brain networks, relative to those in the phenotype characterized by Relative Favorable Recovery (Cluster 4). Those with Predominant Mood and Behavioral Deficits (Cluster 3) exhibit lower volumes in dorsal attention and visual networks, relative to those with favorable recovery.
As one of the few multimodal investigations into the underlying pathophysiology of chronic TBI, the LETBI study, which recently expanded to include military veterans with Department of Defense funding, has the potential to greatly improve the precision and nuance of TBI classification. Already, the evaluation of postmortem brain specimens collected from study participants has begun to elucidate the ex vivo neuroimaging and neuropathological correlations with in vivo neurobehavioral phenotypes. Together, this work promises to fundamentally advance diagnosis and treatment of individuals with distinct clinical symptom patterns following TBI.
Novel Methods Are Expanding Access to Specialized Care for People With TBI: Translating Research Into Practice
It typically takes 17 years for a new intervention to reach clinical settings, but BIRC investigators are committed to narrowing that gap. For example, Maria Kajankova, PhD, and her team developed and validated an online emotion regulation (EmReg) intervention for individuals with TBI, and the demand for this intervention has far exceeded the number of clinicians trained to provide it.
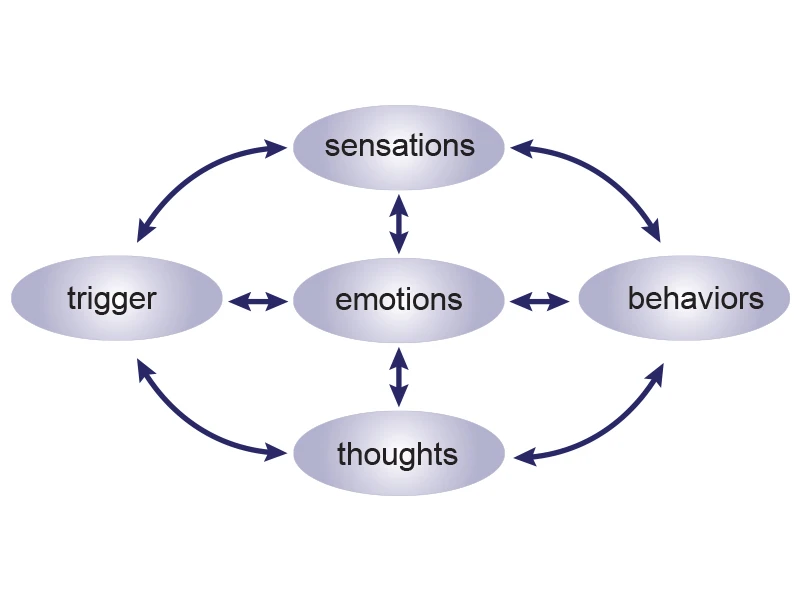
Figure 4. The team developed and validated an online emotion regulation (EmReg) intervention for individuals with TBI.
Emotional dysregulation, which can cause impulsive behaviors, angry outbursts, and feelings of being overwhelmed, is one of the most common and life-altering consequences of TBI. Poorly regulated responses can erode relationships, work performance, and daily life. The EmReg intervention helps individuals effectively regulate their emotions using the emotional cycle, leading to improvements in emotional and functional outcomes (See Figure 4).
Today, Mount Sinai researchers are training clinicians nationwide ...
... on an evidence-based rehabilitation intervention for TBI patients.
The Mount Sinai researchers soon realized, however, they would need to train more clinicians to deliver the intervention—and measure the effectiveness of the intervention delivered by these newly trained clinicians.
With funding from the National Institute on Disability, Independent Living, and Rehabilitation Research, the team is conducting a hybrid type III implementation-effectiveness trial, which aims to determine if and how structured training influences uptake and delivery of the EmReg intervention by community-based clinicians.
Today, the team is training clinicians nationwide, allowing them to answer important questions about how evidence-based rehabilitation interventions can be translated into the community and measuring intervention effectiveness in real-world settings. This work has the potential to dramatically increase access to specialized care for people living with TBI, an underserved population that historically faces innumerable barriers to care.
Featured

Kristen Dams-O'Connor, PhD
Director of the Brain Injury Research Center of Mount Sinai, and Professor, Rehabilitation Medicine, and Neurology

Carrie Esopenko, PhD
Associate Professor, Rehabilitation and Human Performance

Maria Kajankova, PhD
Assistant Professor, Rehabilitation and Human Performance

Raj G. Kumar, PhD, MPH
Assistant Professor, Rehabilitation and Human Performance