Additionally, the amount of time that a brain can be monitored in each patient is often quite short, as recording sessions are limited by the treatment that the individual is receiving. However, the greatest limitation of human studies is the difficulty in gaining causal insight into underlying mechanisms. To circumvent these issues and to provide insight into the function of the healthy human brain, researchers turn to animal models, in which brain activity can be manipulated and the consequences assessed.
To ensure that what is learned from animal models has the best chance of translating to the human brain, scientists at The Friedman Brain Institute (FBI) work with nonhuman primates, such as macaque monkeys. Unlike the brains of rodents, such as rats and mice, macaque brains share the same complex organization as the human brain, with the parts of the brain involved in higher cognitive functions greatly expanded and highly differentiated. One example is regions of the prefrontal cortex, which are implicated in most forms of neurological and psychiatric illnesses yet exist in extremely rudimentary forms in rodents.
In a recent study, scientists taught macaques to perform a task that requires them to remember information over short periods of time. This process, known as working memory, is what we all use when we try to remember a new phone number.
In the Lipschultz Center for Cognitive Neuroscience, the laboratories of Peter H. Rudebeck, PhD, and Erin L. Rich, MD, PhD, have pioneered the use of high-density and chronic recording methods in macaques that enable the activity of hundreds of individual neurons to be measured while the monkeys are engaged in situations that require them to use human-like reasoning. This work complements and extends the research being conducted in humans undergoing intracranial monitoring (see article).
In a recent study, the Rich lab taught macaques to perform a task that requires them to remember information over short periods of time. This process, known as working memory, is what we all use when we try to remember a new phone number and it is critically affected in Alzheimer’s disease. Just like humans, the macaques use strategies to help them retain information in working memory.
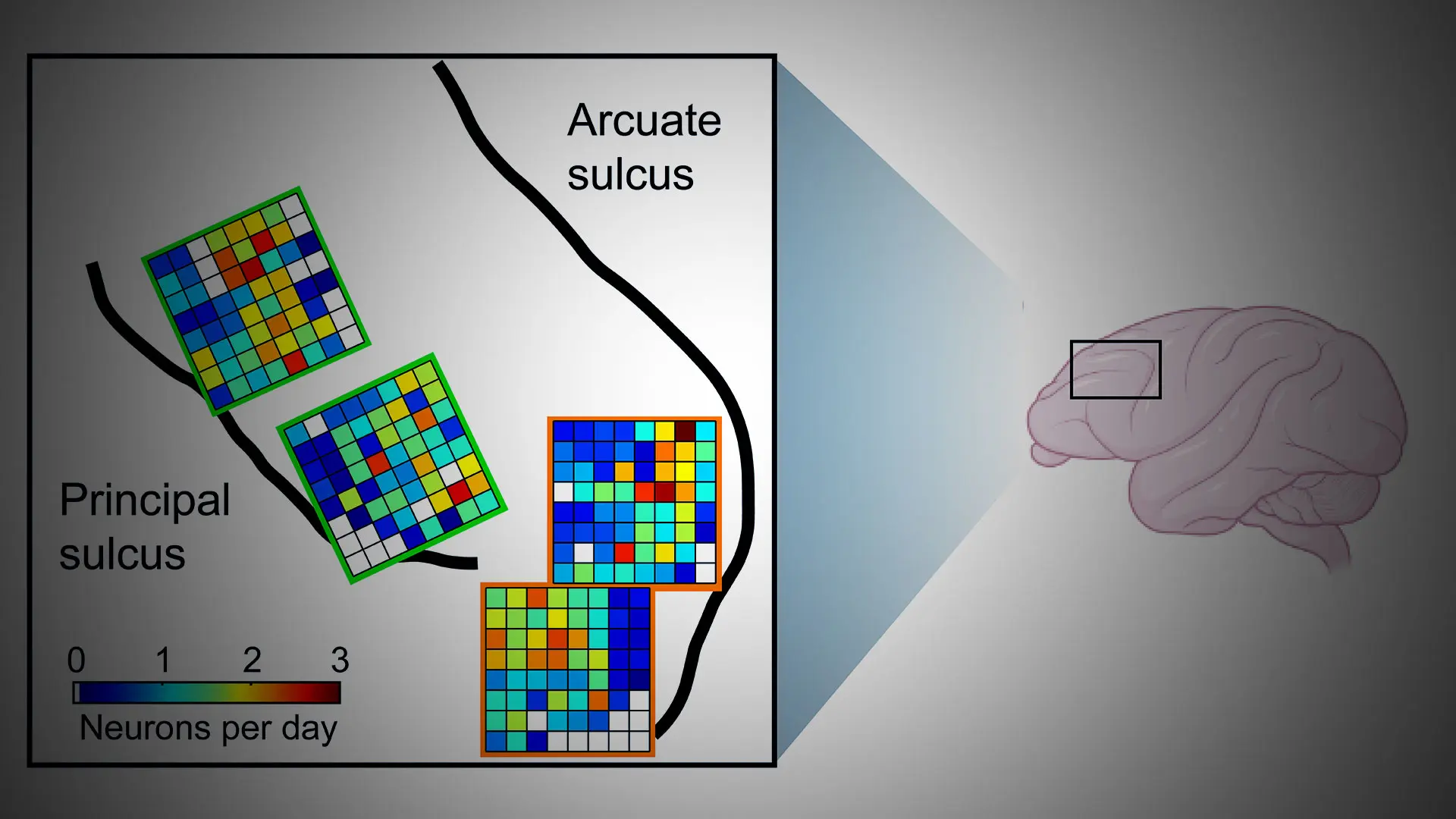
For example, they group information into easily remembered pieces, known as “chunks,” just like their human counterparts. Using the latest methods, the Rich lab has simultaneously measured the activity of hundreds of neurons in the dorsolateral prefrontal cortex, which is known to support working memory (see Figure 1). Lab members found that neurons change their activity when macaques are chunking information together in working memory. Ongoing research is aimed at figuring out how dorsolateral prefrontal cortex interacts with other parts of the brain to support this type of higher-level reasoning.
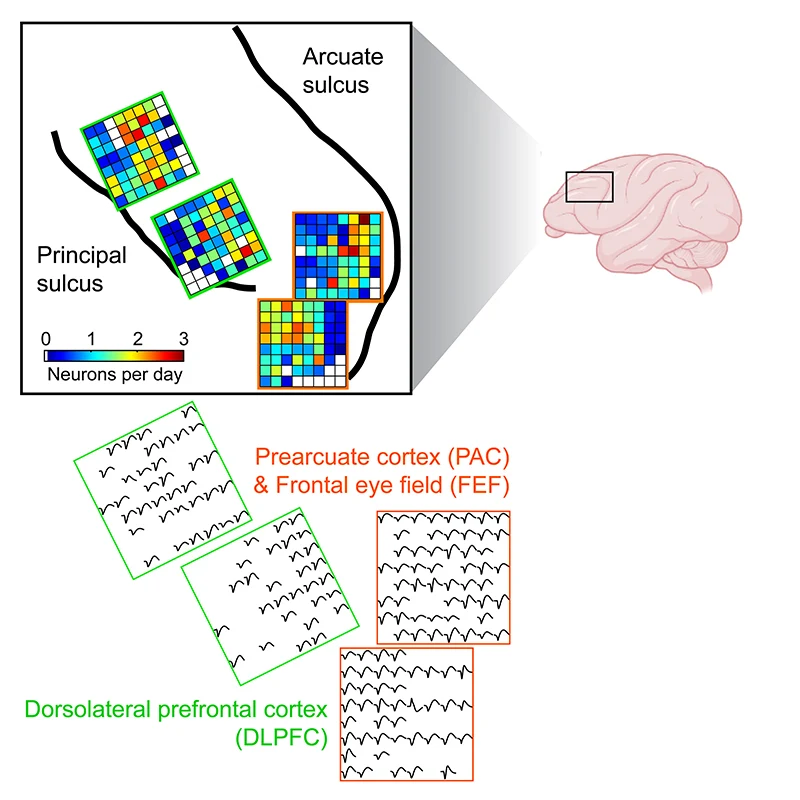
Figure 1. High-density neuronal recordings from macaque dorsolateral prefrontal cortex. Credit: Erin Rich, PhD, and Feng-Kuei Chiang, PhD
In another study, the Rudebeck lab monitored the activity of hundreds of neurons across the brain of macaques for many months. Each day, the macaques made choices between different fruit juices based on their preferences. Just like humans, the macaques’ preferences for the two juices they were offered changed from day to day. When the researchers looked at how these changes in preference related to neural activity, they found that neurons in the ventrolateral prefrontal cortex were signaling the change in preference on a day-to-day and even minute-to-minute basis.
These results are exciting because they raise the possibility of studying phenomena that vary significantly across days, weeks, months—and even, potentially, years—such as moods or motivational states. This work is being followed up by using virally mediated chemogenetic approaches to causally manipulate the inputs to the ventrolateral prefrontal cortex as macaques make decisions.
Working with macaques not only allows researchers at the FBI to characterize how neural activity supports cognition with high precision, but it also allows current and emerging treatments for neurological and psychiatric disorders to be studied from the level of behavior to molecules.
Such information is essential for refining neuromodulation therapies for human brain disorders. Indeed, a major collaborative effort at the FBI uses macaques to study how deep brain stimulation (DBS) for treatment-resistant depression impacts neural activity across distributed brain circuits.
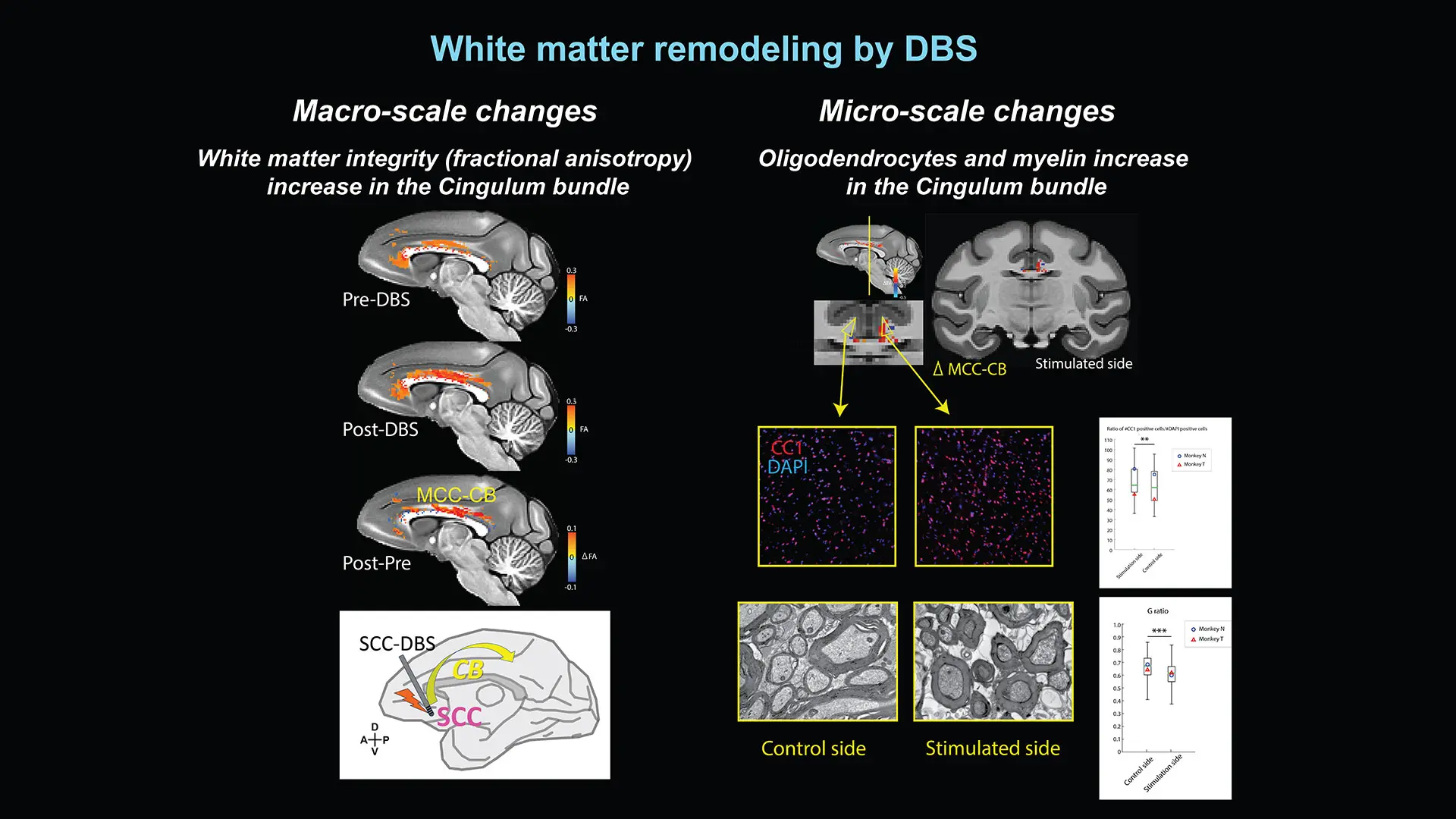
Figure 2. The effect of subcallosal ACC deep brain stimulation on white matter in the brain. Credit: Satoka Fujimoto, PhD
Using exactly the same approach employed in human patients with depression, Dr. Rudebeck, working in concert with Helen S. Mayberg, MD, Founding Director of the Nash Family Center for Advanced Circuit Therapeutics, has discovered that DBS targeted to ventral prefrontal cortex not only rebalances brain-wide patterns of neural activity but also leads to both macro- and microscopic changes in the white matter connections between brain areas (see Figure 2). Such discoveries reinforce the essential importance of research with macaques for advancing our understanding of the human brain in both health and disease.
Featured

Peter H. Rudebeck, PhD
Associate Professor of Neuroscience, and Psychiatry, Icahn School of Medicine at Mount Sinai

Erin L. Rich, MD, PhD
Associate Professor of Neuroscience, Icahn School of Medicine at Mount Sinai

Helen S. Mayberg, MD
Founding Director, Nash Family Center for Advanced Circuit Therapeutics, and Professor of Artificial Intelligence and Human Health, Neurosurgery, Neurology, Neuroscience, and Psychiatry