Dr. Malaspina is Professor of Psychiatry, Neuroscience, and Genetics and Genomic Sciences.
This circuitry involves the gut microbiome, the sympathetic and parasympathetic nervous systems, and circulating molecules of the body’s immune system. While one major focus for understanding schizophrenia is its genetic underpinnings (see article on the genetic basis of schizophrenia), a large portion of disease risk falls on nongenetic factors.
Such factors include social determinants of health, such as older age of parents, prenatal conditions (for example, stress and malnutrition), adverse complications during birth, early child and adolescent development (for example, physical and sexual abuse, neglect, social exclusion, drugs of abuse), and general working and living conditions. There is even evidence that exposures of ancestors to environmental stressors can cross generations and influence the risk for mental illness in other family members, although the underlying epigenetic mechanisms that mediate such effects remain poorly understood.
Another focus for scientists: nongenetic factors in schizophrenia risk
How do we explain that so many factors are related to schizophrenia? One possibility is that different exposures cause schizophrenia through diverse toxic pathways. An alternate explanation is that schizophrenia and related psychoses—which represent heterogeneous syndromes of diverse etiologies—are a consequence of the over-activation of one or more highly conserved threat response pathways, which evolved in vertebrates and later mammals to enhance an individual’s survival in the face of environmental and social dangers.
We know, for example, that prenatal adversity can program lifelong changes in gene expression in several organs to enhance survival if food is scarce, but it also can predict preterm birth, as well as cardiometabolic and brain disorders in humans.
Another pathway activated by social threats increases inflammatory gene expression and shifts blood cell formation toward the production of myeloid cells, which play crucial roles in pathogen defense, immune regulation, and tissue repair. Unfortunately, this immune activation also increases the risk for autoimmune disorders, allergies, and inflammatory diseases, including several brain disorders. Some of the genetic variations that are most strongly associated with schizophrenia encode proteins involved in immune functioning, and they may be responsible for ramping up these systems. Likewise, advanced paternal age, which is another psychosis risk factor, predicts rare and de novo gene variants with shorter cell cycles and proinflammatory effects.

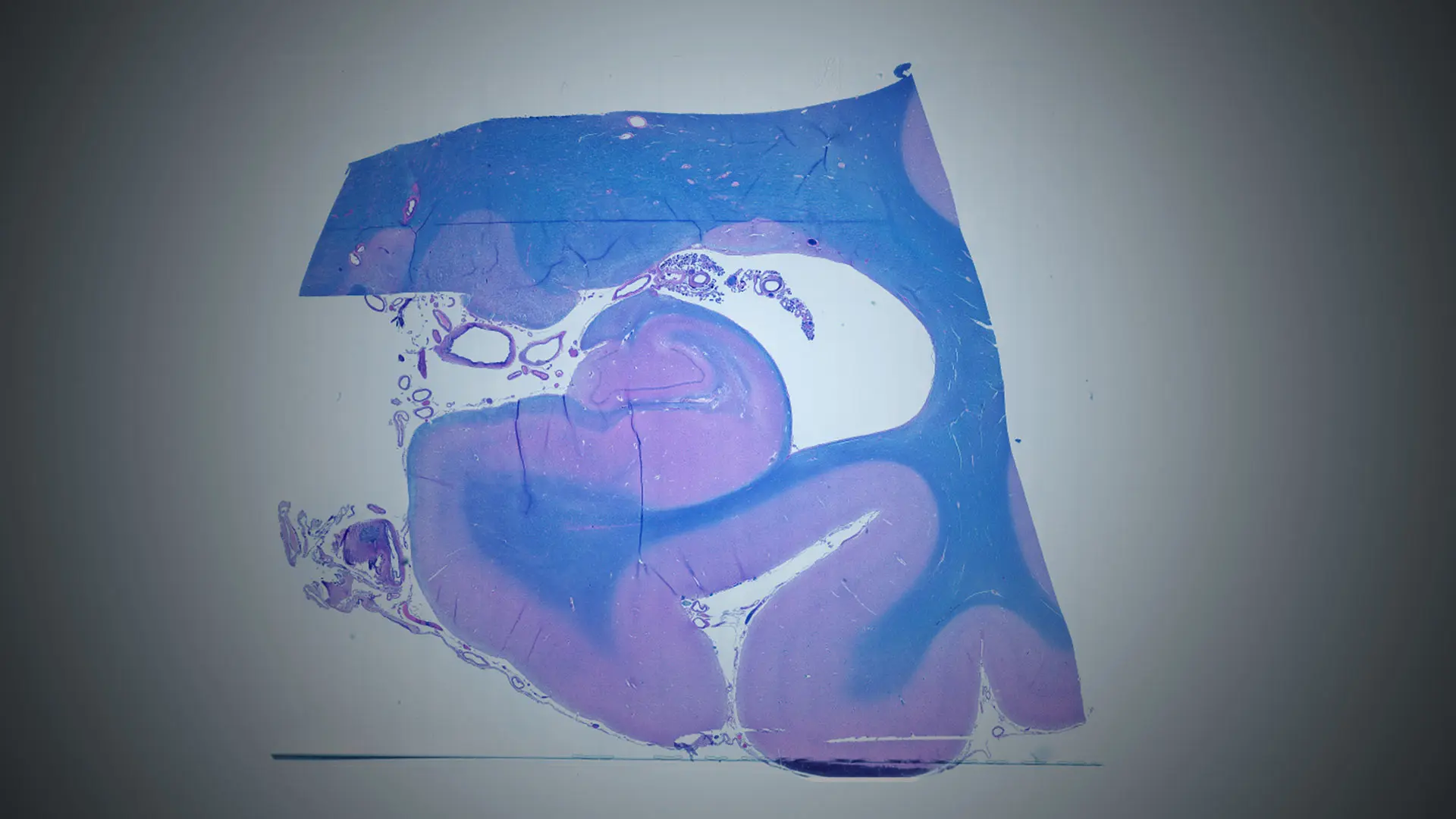
Our studies, in particular, link inflammatory changes within the hippocampus in persons with psychosis to sympathetic and parasympathetic reflexes involved in blood flow regulation and the gut microbiome, changes that are thought to contribute eventually to derangements in brain function (see Figure above). Key team members involved in this work are Jose Clemente, PhD; Bridget Mueller, MD, PhD; Jessica Robinson-Papp, MD, MS; and Jakleen Lee, an MD/PhD student at the Icahn School of Medicine at Mount Sinai.
Understanding that social determinants underlie a significant proportion of the risk for schizophrenia and other severe forms of mental illness has one significant benefit—the promise that prevention can be derived from policy changes. In the meantime, having teams of scientists illuminate possible biomarkers for threat response pathways that contribute to the pathophysiology of these disorders will set the stage for targeted treatments—and, eventually, preventive measures.
Featured

Dolores Malaspina, MD, MSPH
Professor of Psychiatry, Neuroscience, and Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai

Jessica Robinson-Papp, MD, MS
Professor of Neurology, Icahn School of Medicine at Mount Sinai

Bridget Mueller, MD, PhD
Assistant Professor of Neurology, Icahn School of Medicine at Mount Sinai

Jose Clemente, PhD
Associate Professor, Genetics and Genomic Sciences, and Medicine (Clinical Immunology), Icahn School of Medicine at Mount Sinai

Jakleen Lee
MD / PhD student, Icahn School of Medicine at Mount Sinai