The search for such genes that are related to schizophrenia is justified by the decades-old knowledge that the risk for schizophrenia and related psychotic disorders is roughly 60 percent genetic. This places schizophrenia as a highly heritable condition, more heritable, for example, than hypercholesterolemia, high blood pressure, type 2 diabetes, and many forms of cancer. Yet, until recently, it has been difficult to identify the genes that confer this risk.
~60%
The genetic risk for schizophrenia and related psychotic disorders
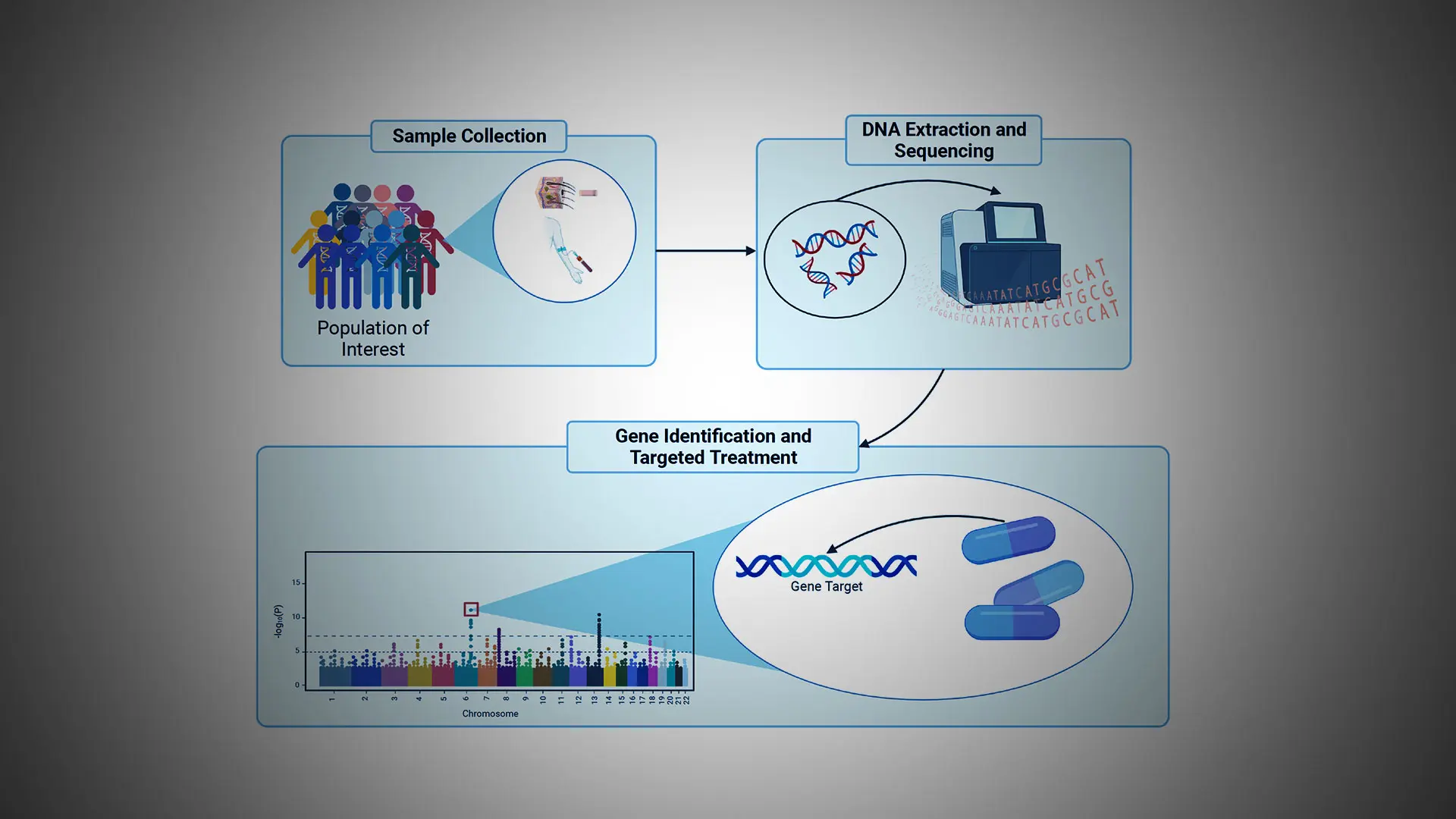
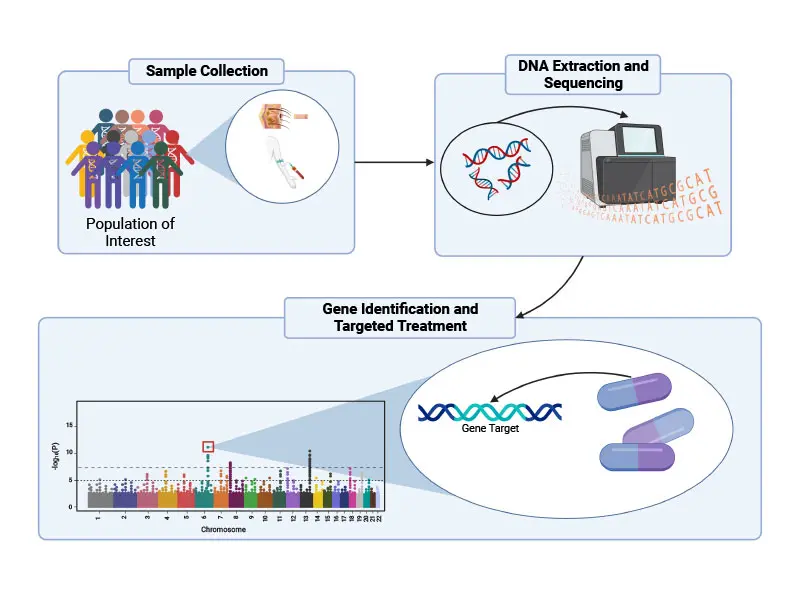
Over the past decade, immense progress has been made in mapping the genetic architecture of schizophrenia. These advances were facilitated by the development of chip-based technologies that permit rapid and relatively inexpensive genotyping of individuals and through the analysis of tens of thousands of individuals with schizophrenia (and age- and sex-matched control subjects) that provides the statistical power required for gene identification.
Further advances were enabled by the advent of next-generation sequencing technology that makes it possible to sequence the entire genome of individual humans (each genome contains a unique linear sequence of ~3 billion nucleotide base units) or the small subset of the genome (~1%) referred to as the exome, which represents only those DNA nucleotide base units that encode proteins.
By use of these various methods, the field has now identified several hundred genetic variations (differences in DNA sequence) that are associated with increased risk for schizophrenia, an effort that was spearheaded internationally in part by the late Pamela Sklar, MD, PhD, and her colleagues at Mount Sinai.
Despite this progress, however, fundamental challenges have remained. Most particularly, additional investigation is necessary to better understand the neurobiological mechanisms by which genetic variants increase the risk for schizophrenia.
For example, one of the ways a person’s genetics can increase individual risk for schizophrenia is through what are called “rare damaging genetic variants” (RDGVs). As the name suggests, RDGVs are sequences in a person’s genome that do not appear frequently in the genomes of other people—they are “rare”—and they render the gene in which the sequence occurs dysfunctional, or “damaging.”
Searching the genomes of 100,000+ patients with severe mental illness
Mount Sinai’s current efforts in schizophrenia genetics, led by Alexander Charney, MD, PhD, and his colleagues, involve searching the genomes of more than 30,000 individuals with schizophrenia for RDGVs. While the group has identified 12 genes that may promote schizophrenia through RDGVs, they have also learned that there are likely many more such genes, according to a new study that is accepted for publication in early 2023. To identify these new genes, the team is now searching the genomes of more than 100,000 additional patients with severe mental illness.
Sequencing the genomes of 150,000 individuals worldwide
Toward this end, the Jeff and Lisa Blau Adolescent Consultation Center for Resilience and Treatment (see Blau Center article) has separately partnered with the Regeneron Genomics Center (RGC) and two other academic medical centers, the University of North Carolina and Cardiff University, in Cardiff, Wales, to sequence the genomes of 150,000 individuals of all races and ethnicities from across the world with severe psychiatric disorders.
Importantly, a major part of this study—the genetic sequencing step—will be conducted by RGC without cost to the academic centers, an investment of approximately $60 million. The data generated will be made available to academic researchers worldwide to advance discoveries as rapidly as possible.
In addition to these initiatives, genes discovered by the Mental Illness Genetics Moonshot Study will be further studied by Blau Center researchers. This effort is part of the newly launched Mount Sinai Million Health Discoveries Program through which the genome sequences of 1 million consenting Mount Sinai Health System patients will be obtained over the next five years or so.
The Mental Illness Genetics Moonshot Study will focus specifically on how genes associated with the risk for schizophrenia affect the clinical presentation, treatment, and long-term prognosis for this illness.
Examples of this approach have been successful in other brain disorders—most notably in Alzheimer’s disease—in which relatively rare genetic variants, such as those found in the gene that codes for amyloid proteins that accumulate in the brains of people with Alzheimer’s disease, have been shown to cause the disease in specific patients and their extended families. In a similar way, Blau Center investigators, as part of the Mental Illness Genetics Moonshot Study, will start by examining more deeply how the 12 genes identified to date affect schizophrenia.
As one example, the team has recently identified a family with a specific RDGV in a gene called GRIA3, which encodes a type of glutamate receptor. Glutamate is the major excitatory neurotransmitter in the brain. The RDGV in GRIA3 seems to be a cause of schizophrenia across three generations of the family (see Figure above). Blau Center investigators are now building a relationship with the family so they can work with them to gain a better understanding of how this RDGV possibly causes their illness.
In contrast to these RDGVs, most of the several hundred identified genetic risk factors for schizophrenia exert a very small effect on overall risk for the illness. This poses a major challenge in understanding how hundreds of genetic variations come together in a given individual to cause schizophrenia. Understanding the mechanisms behind such “polygenic risk” is another major focus of Mount Sinai investigators.
Finally, a large fraction of these genetic risk factors do not occur within the coding region of the genome, meaning that the variations do not affect the proteins expressed by the affected gene. Instead, many schizophrenia-associated gene sequence variations occur in so-called noncoding regions of the genome where they are thought to affect the overall structure of DNA in the cell nucleus, which in turn affects expression patterns of multiple genes. This is another robust concentration of research for Mount Sinai’s scientists (see article on the transcriptomic and epigenomic basis of schizophrenia).
Together, these efforts will, at long last, bring the study of schizophrenia into the modern era. Knowing how genes contribute to the illness will provide multiple paths forward to develop a truly personalized approach to treating schizophrenia and other severe mental illnesses.