Moreover, schizophrenia and other mental disorders are especially complex with respect to etiology. As just one example: Variations at many hundreds of genomic loci are implicated in disease risk—with each variant in nearly all of the cases that have been identified to date making an extremely small contribution. This poses a formidable challenge for a deeper understanding of the disease mechanisms that underlie schizophrenia and other psychiatric” syndromes. Perhaps unsurprisingly, very few advances in treatment and prevention have been made over the course of the last 50 years.
Our scientists are leading national and world efforts to study…
…postmortem brain tissue from thousands of subjects.
Today, multiple investigators from Mount Sinai’s Department of Psychiatry and The Friedman Brain Institute (FBI) are leading efforts in large national and international consortia, including the CommonMind Consortium and psychENCODE Consortium, to study postmortem brain tissue from thousands of subjects diagnosed with serious mental illness, including schizophrenia. Importantly, this research includes age- and sex-matched control subjects.
At Mount Sinai, these team efforts are being led by Panagiotis (Panos) Roussos, MD, PhD, and Schahram Akbarian, MD, PhD, each a Professor of Psychiatry, and Genetics and Genomic Sciences, at the Icahn School of Medicine at Mount Sinai. The goal is to study variations in the organization and function of the genome associated with a given disease state, such as schizophrenia, at population scale.
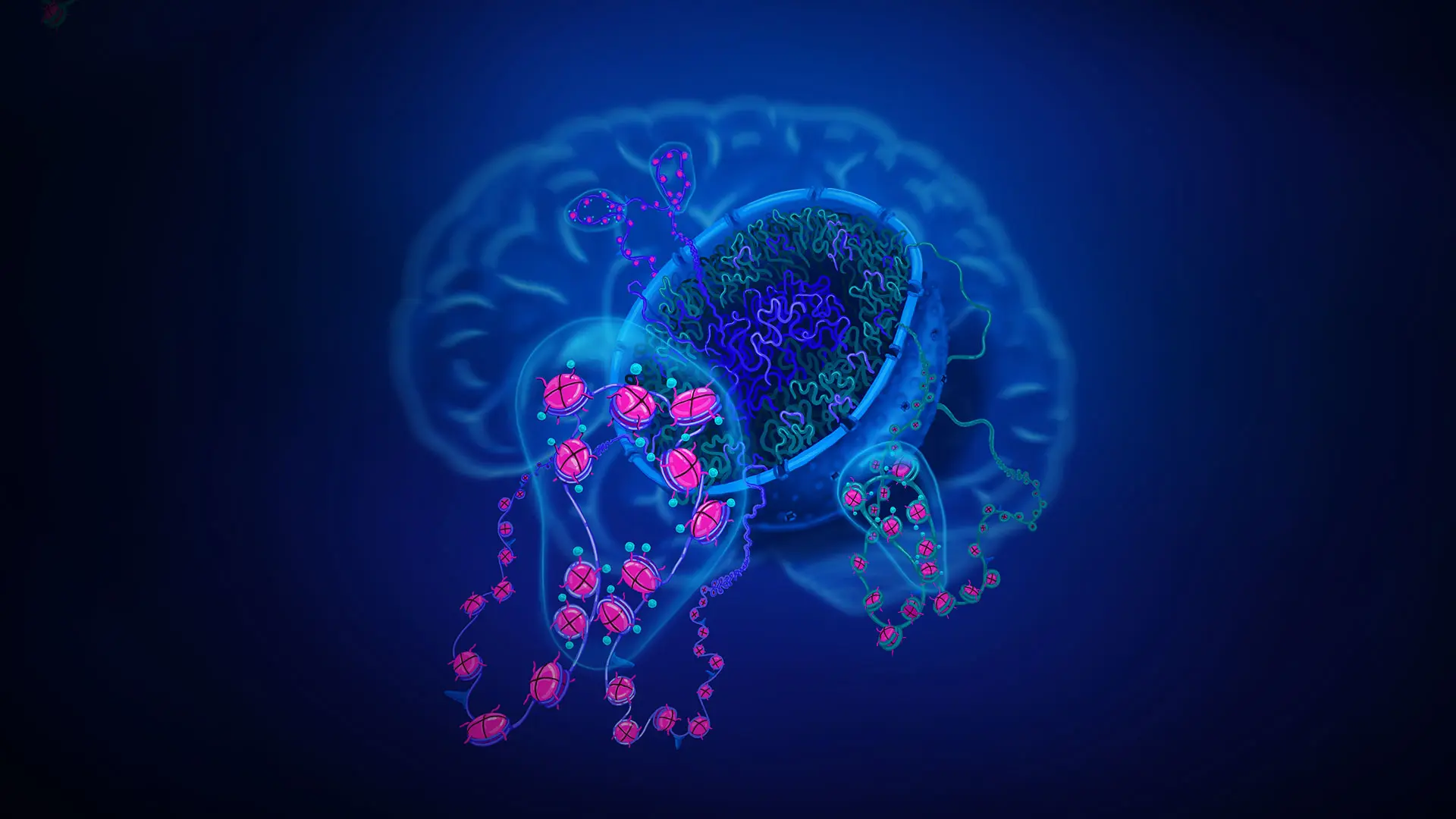
In one project, Mount Sinai investigators are focusing on the so-called "epigenomic landscape" of each chromosome and the way in which DNA and associated histones and other proteins are packaged into the three-dimensional space inside the nucleus of nerve cells in the human brain. This "3D genome" organization, characterized by a method called “Hi-C,” is critically important for proper functioning of the linear DNA sequence because active chromatin sites (the areas where genes are turned on) require an intranuclear "neighborhood" that is chemically and molecularly very different from the neighborhood that defines inactive chromatin (see Figure).
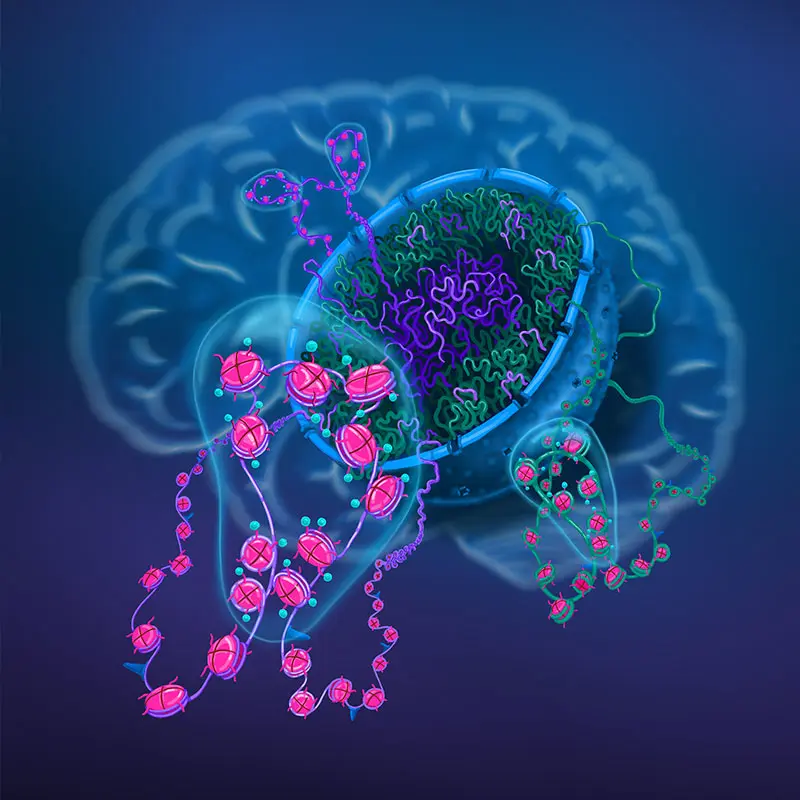
Artist's illustration of chromosomal organization in 3D inside the nucleus of brain cells.
The chromosomal material located toward the center of the nucleus (shown in purple) tends to become dysregulated in schizophrenia and related conditions, while the material in the nuclear periphery (shown in green) is less frequently affected. The chromosomal material in the brain is organized into thousands of functional domains (shown as bubbles) defined by chromosomal loopings and chemically modified DNA-histone protein complexes. It is possible that, on a genome-wide scale, up to 10 percent of chromosomal domains are affected in the brains of individuals with schizophrenia. Image by Jessica S. Johnson, Icahn School of Medicine at Mount Sinai
A further complication is that the 3D genome varies dramatically across different types of nerve cells in the brain, and also from several types of non-neuronal cells, which obligates scientists to perform studies in a cell type-specific manner across numerous brain regions. These maps of the 3D genome are then correlated with global measures of gene expression (so-called transcriptomic mapping) by use of RNA-sequencing methods, focusing again on individual cell types across several brain regions—and, in some cases, by use of single-cell approaches to map expressed transcripts in each individual cell separately.
These are highly innovative studies and, to date, these approaches have not been applied on this scale to diseased human brain tissue, yet the results that scientists are seeing are already offering deep insights into the genomic underpinnings of serious mental illness.
New approaches have uncovered some deep insights into the genomic landscape.
For example, in a recent study, the Mount Sinai team uncovered—in the context of schizophrenia—that disruptions in the normal epigenomic landscape of chromosomes disproportionally affect neuronal cell populations of the prefrontal cortex (a region of brain that is highly developed in the primate lineage and mediates aspects of working memory and executive function).
Moreover, these disruptions affect hundreds of genomic regions that are spread across many different chromosomes. Unexpectedly, many of these chromosomal hotspots for schizophrenia-associated changes share the same intranuclear neighborhoods buried deep inside the center of the cell nucleus where some of the most actively expressed genes of the human brain are housed.
Intriguingly, some of the same chromosomal sites that display changes in chromatin activity in the prefrontal cortex of individuals with schizophrenia exhibit active epigenomic patterning during normal human brain development, raising the possibility that these are "seed points" for genomic disorder that ultimately, decades later, may manifest as schizophrenia.
A major goal of current research by FBI scientists is to understand the fundamental abnormality that is causing such widespread derangements in 3D chromatin structure across so many chromosomal regions within prefrontal cortical neurons selectively.
For example, FBI investigators intend to explore the use of artificial intelligence tools to delineate the mechanisms underlying the coordinated genome-scale dysregulation of chromosomal organization in schizophrenia and how such mechanisms relate to the genetic risk burden of each specific individual (see article on the genetic basis of schizophrenia). These efforts promise to yield bold discoveries that will eventually allow better prediction models of disease prevention and treatment.
Featured

Panagiotis (Panos) Roussos, MD, PhD
Professor of Genetics and Genomic Sciences, and Psychiatry, Icahn School of Medicine at Mount Sinai

Schahram Akbarian, MD, PhD
Professor of Psychiatry, Neuroscience, and Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai