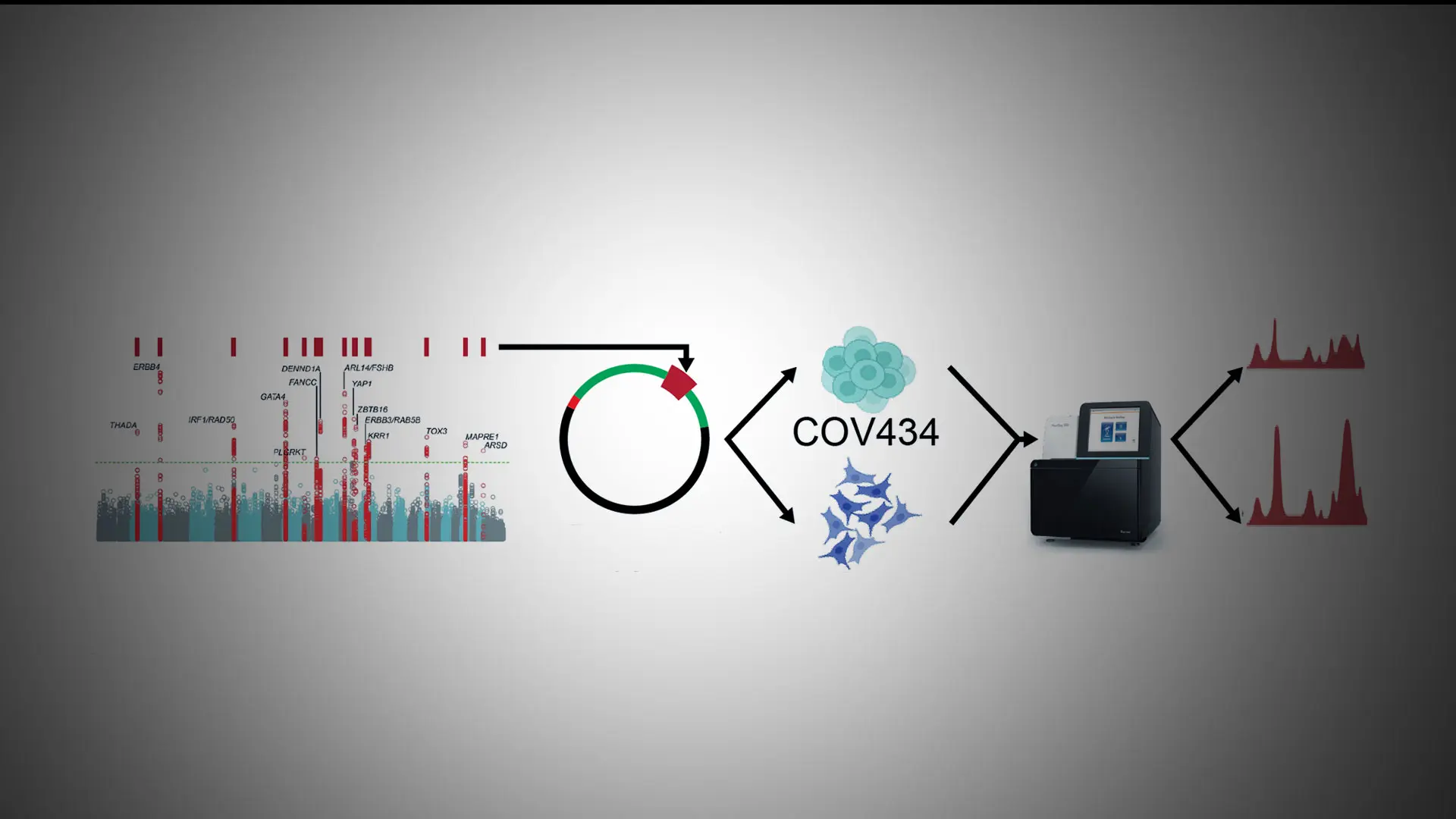
A Mount Sinai team has identified regions of DNA associated with testosterone production in women with polycystic ovary syndrome (PCOS), bringing the field a step closer to determining the molecular causes of the condition. The research was led by Andrea Dunaif, MD, Chief of the Division of Endocrinology, Diabetes and Bone Disease, Icahn School of Medicine at Mount Sinai.
PCOS, which affects 10 percent to 15 percent of the menstruating population, is characterized by increased testosterone production by the ovaries and, sometimes, the adrenal glands. It frequently disrupts ovulation, impairs fertility, and increases the risk for developing type 2 diabetes and cardiovascular disease. It also causes symptoms such as increased facial hair growth and acne, thereby diminishing quality of life. Genome-wide association studies (GWAS) have identified about 20 different gene regions that appear to play a causal role in PCOS via neuroendocrine, reproductive, and metabolic pathways. The Mount Sinai team is investigating how these pathways are disrupted in PCOS.
“It has been difficult to determine the function of the gene regions that are mapped in GWAS, because the significantly associated single nucleotide variants are rarely the disease-causing variants themselves, but rather are tagging regions of the genome containing the actual causal variants. Further, most GWAS variants are within noncoding portions of the genome and have modest effects on phenotype, making their function difficult to interrogate,” says Dr. Dunaif, a world leader in the study of PCOS and the Lillian and Henry M. Stratton Professor of Molecular Medicine. “It has been exceptionally challenging to determine how genetic variation results in PCOS.”
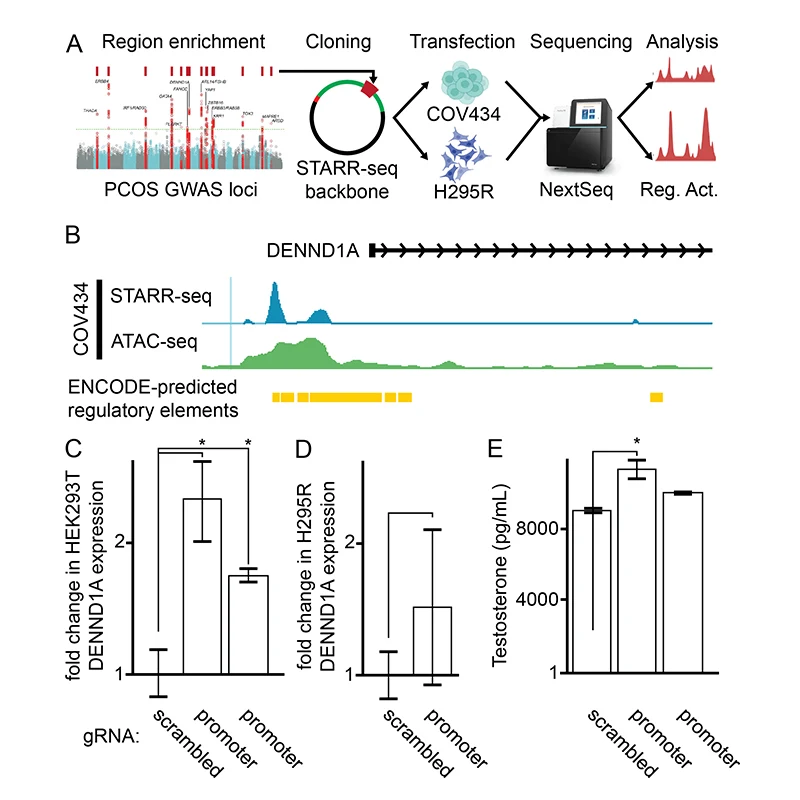
Traditional targeted resequencing failed to identify likely causal variants within PCOS GWAS loci, but these studies were underpowered. In collaboration with Tim Reddy, PhD, at Duke University, the team used high-throughput reporter assays to locate regions in FSHB, GATA4, and DENND1A that either increase or decrease gene activity. As a proof-of-concept, they used CRISPR-based epigenetic manipulations to show, for the first time, that several specific regions of DNA sequence within DENND1A increase testosterone production in a PCOS ovarian cell model.
Abstract presented at the symposium "Unraveling the Genetic Determinants of Female Infertility" during the annual meeting of the American Society of Human Genetics.
“This discovery brings us much closer to determining how that gene is disrupted in PCOS and, ultimately, how we might be able to target therapy to decrease testosterone production to treat PCOS.”
Andrea Dunaif, MD
“There is evidence that the DENND1A gene plays an important role in PCOS, but, until now, nobody has been able to map the exact variants in DENND1A that contribute to PCOS. We’ve been able to identify variants in the gene that regulate its activity and result in increases in testosterone production,” Dr. Dunaif says. “This discovery brings us much closer to determining how that gene is disrupted in PCOS and, ultimately, how we might be able to target therapy to decrease testosterone production to treat PCOS.”
These findings were selected for presentation as an oral abstract at the annual meeting of the American Society for Human Genetics, held in November 2023 in Washington, D.C., as part of a platform symposium titled “Unraveling the Genetic Determinants of Female Infertility”. Dr. Dunaif’s team also presented updates to previous work on data-driven classification of PCOS.
Reproductive and metabolic subtypes of PCOS have been identified using hierarchical cluster analysis of phenotypic traits. In contrast to the PCOS phenotypes identified by the current diagnostic criteria, which are genetically similar, these subtypes were associated with novel and distinct genome-wide significant loci, suggesting that the subtypes captured biologically meaningful differences. Now, the team has replicated these subtypes in PCOS cases of Korean ancestry. Further, they have shown that there are shared subtype-specific genetic loci in a transethnic meta-analysis in PCOS cases of European and Korean ancestry.
“In collaboration with Joop Laven, MD, PhD, at Erasmus University Medical Center in the Netherlands, we’ve found that these subtypes are present in regionally and ethnically diverse PCOS cohorts, as well as in cases diagnosed by both the NIH and Rotterdam diagnostic criteria, Dr. Dunaif says. “Further, we’ve shown that a number of additional phenotypic traits not used for clustering differ significantly among the subtypes and align with the pathways we think are disrupted. We are continuing our collaborations with Drs. Reddy and Laven to map genes associated with PCOS subtypes and determine their contribution to PCOS pathogenesis via functional and perturbational assays.”
Featured

Andrea Dunaif, MD
Chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes and Bone Disease, and the Lillian and Henry M. Stratton Professor of Molecular Medicine