The Diabetes, Obesity and Metabolism Institute (DOMI) at the Icahn School of Medicine at Mount Sinai made further outstanding progress in 2023 in the study of diabetes, obesity, and metabolic syndromes. Under the leadership of Director Andrew F. Stewart, MD, the Institute in 10 years has grown from about $1 million a year in grant support to $8 million, from sources including the National Institutes of Health (NIH), the United States Department of Defense, and the Juvenile Diabetes Research Foundation.
The Institute continued its pioneering research in beta cell regeneration in Dr. Stewart’s laboratory, and a cohort of physician-scientists at DOMI made strides in basic and translational science. Among them are Prashant Rajbhandari, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease), who led a team that discovered a family of molecules in the breast called “mammokines” that contribute not only to normal mammary biology, but to overall fat cell physiology and energy balance control. Working in a mouse model, Dr. Rajbhandari “found control of metabolic rate in the whole body by a gland that nobody ever anticipated might have any relevance to metabolism,” says Dr. Stewart, the Irene and Dr. Arthur M. Fishberg Professor of Medicine (Endocrinology, Diabetes and Bone Disease).
Emily Gallagher, MD, PhD, Associate Professor of Medicine (Endocrinology, Diabetes and Bone Disease), is continuing her study of the mechanisms linking elevated triglyceride levels and triple-negative breast cancer, funded by a $2.5 million, five-year grant from the NIH. In addition, in 2023 she was a senior author in a study of multiple myeloma and diabetes, published in Blood Advances.
Dr. Stewart expressed pride and admiration for all of DOMI’s researchers. Three in particular are relatively new to their fields, but are making significant contributions. “These are bright, young, next-generation people who have good stories to tell about science,” he says.
One of them, Lauryn Choleva, MD, MSc, Instructor in the Division of Pediatric Endocrinology and Diabetes, took a winding path to the lab. She was originally a dietitian. “Everybody she saw was a diabetic and overweight, and she was trying to tell people what they should eat,” Dr. Stewart says. “Then she realized, ‘It’s hard to change eating behavior, so I’ll go to medical school so I can treat diabetes.’” After earning her MD, she came to Mount Sinai as a pediatrics resident, and then an endocrinology fellow. A rotation in Dr. Stewart’s lab led her to basic science, working with his team in beta cell regeneration. He says that Dr. Choleva is something of a rarity, a scientist who regularly sees patients—in her case, mainly children with diabetes. “There aren’t enough physician-scientists in the United States,” Dr. Stewart says, “We used to be the dominant people in the world of translational research, and we are not anymore.” Dr. Choleva in February 2024 published her first basic science paper in the journal Endocrinology, described below.
The second early career scientist at DOMI is Sharon Baumel-Alterzon, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease), who came to work at the lab of Donald Scott, PhD, as a postdoctoral fellow. Her background was in basic science, studying the biology of amoeba and trichomonas. “Now she has been reinvented as a beta cell biologist, and already has published several important papers,” Dr. Stewart says. In May 2022, she published a paper in Diabetes that describes a pathway that regulates beta cell survival. “This is a new pathway that is likely to participate in an important way in beta cell failure in people with type 2 diabetes,” Dr. Stewart says. In January 2024, she published another paper in Diabetologia describing how metabolic stress injures beta cells in type 2 diabetes.
The third scientist is Romina Bevacqua, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease). Dr. Bevacqua began her career in animal reproductive biology in Argentina. She did postdoctoral research at Stanford University in human stem cell embryology, then joined Mount Sinai. Dr. Bevacqua is doing advanced and innovative work with CRISPR technology, Dr. Stewart says. CRISPR shows great promise in editing genes, such as those associated with sickle cell disease. “The bad news is that you have to do this in cells that are proliferating,” Dr. Stewart says. “And, of course, human beta cells don't replicate. So if you want to do gene editing in human beta cells, it doesn't work. However, Dr. Bevacqua has figured out a way to do that. She can take human islets, turn them into organoids and do gene editing in them. This is advanced technology that is super relevant.”
These scientists, and the entire complement at DOMI, are part of a “great environment” of innovation, Dr. Stewart says. “They are all using different technologies to do different work that is critically important to diabetes research,” he says. “And they are good people; it’s not enough to just be really smart.”
Below, Dr. Choleva, Dr. Alterzon, and Dr. Bevacqua discuss their most significant recent work.

Lauryn Choleva, MD, MSc
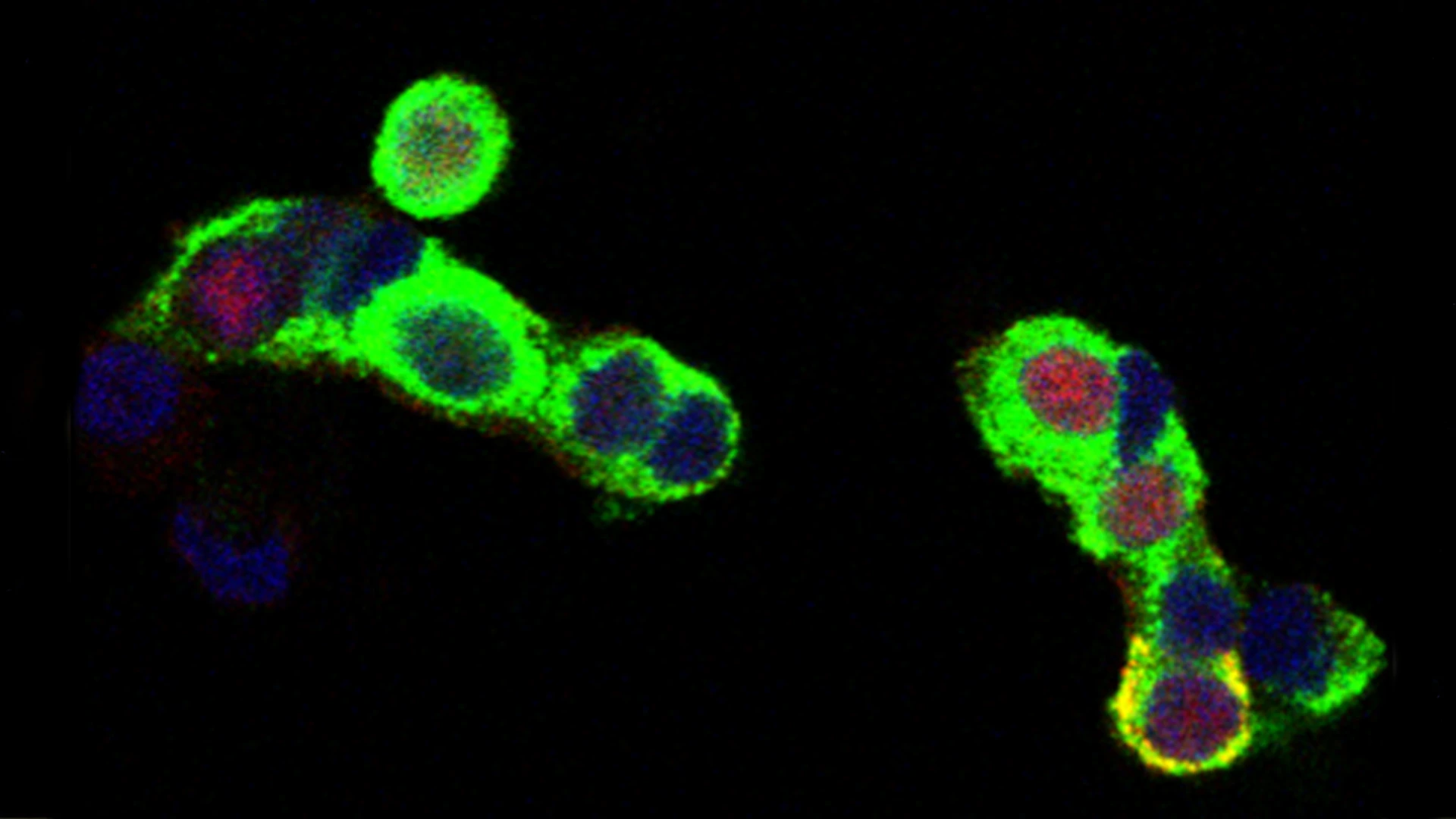
Immunostaining shows expression of p57KIP2 (red) in approximately 50 percent of human beta cells (green).
Takeaway Message: Lauryn Choleva, MD, MSc
More than 280,000 children living in the United States today have diabetes, and the incidence of diabetes is increasing at an alarming rate. My role as a physician-scientist allows me to manage children and adolescents with diabetes in our clinic, while simultaneously working in the laboratory to find a cure. Individuals with both type 1 and type 2 diabetes suffer from reduced beta cell mass and function; as such, identifying therapies to increase the numbers of insulin-producing beta cells and their function is a critical area of research. Specifically, my research focuses on p57KIP2, a cell cycle inhibitor, whose functional role in pancreatic beta cells remains unknown. Our lab has identified harmine, a small molecule of the DYRK1A inhibitor class that induces human beta cells to replicate. Remarkably, harmine causes repression of p57KIP2 expression in beta cells, contributing to their proliferation.
Additionally, mutations in CDKN1C, the gene encoding p57KIP2, are responsible for several rare pediatric endocrine syndromes, including Beckwith-Wiedemann syndrome, the focal variant of congenital hyperinsulinism, Russel-Silver syndrome, and IMAGe syndrome.
Despite its central role in pediatric disease and pancreatic beta cell biology, very little is known about the function of p57KIP2 in any cell type, including the human beta cell. We believe that p57KIP2 has as yet unknown functions that extend beyond cell cycle control and are unique to the human beta cell. Our work will provide a link to the human uniqueness of CDKN1C expression in beta cells, and shed light on the pathogenesis and underlying mechanisms of several pediatric endocrine syndromes. As a pediatric endocrinologist, understanding the molecular pathways behind the disorders affecting the patients I treat is integral to translating laboratory discovery into patient care.

Sharon Baumel-Alterzon, PhD
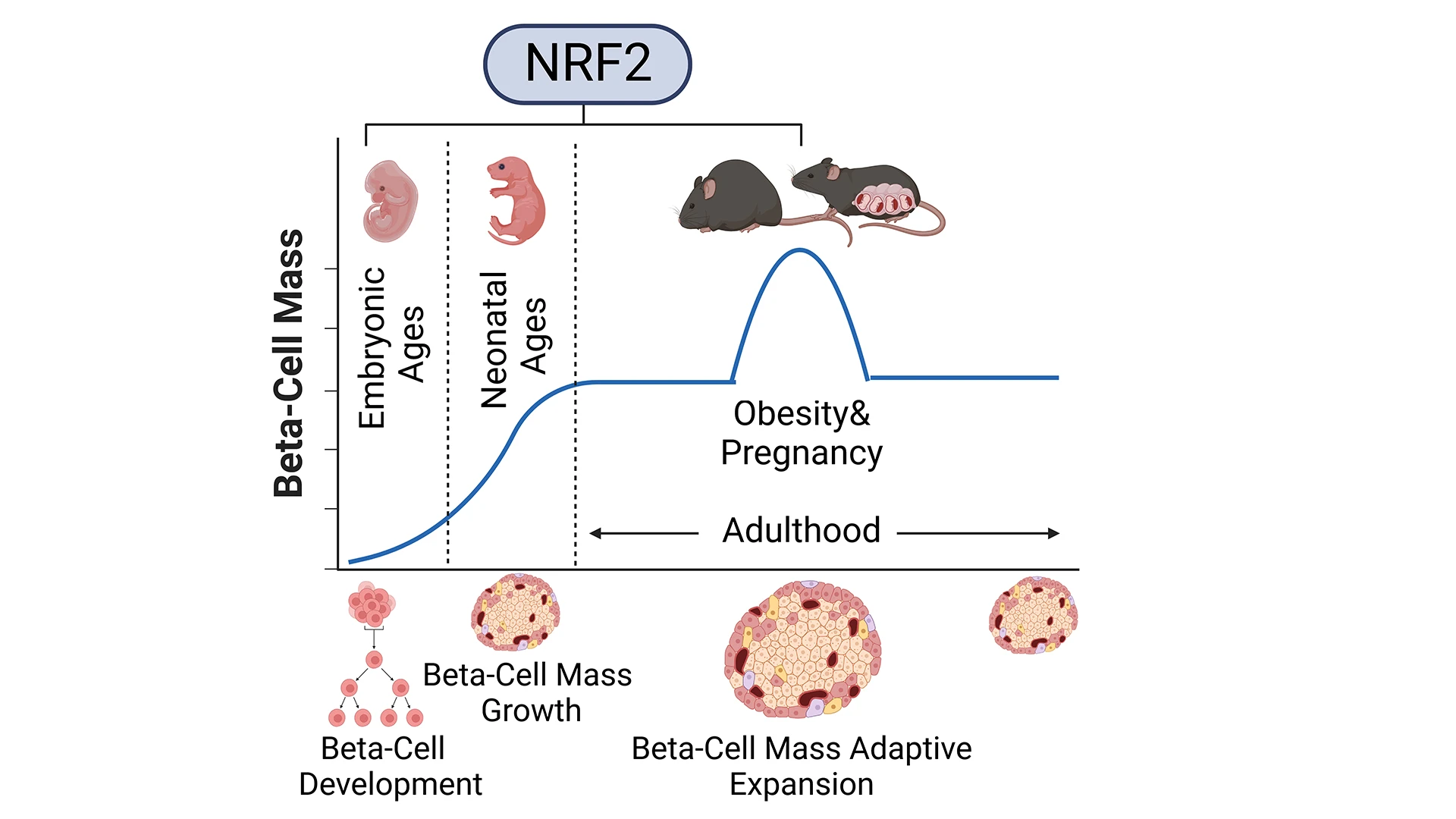
Studying the role of NRF2 in the expansion of beta cell mass during embryonic development, neonatal growth, obesity, and pregnancy.
Takeaway Message: Sharon Baumel-Alterzon, PhD
All forms of diabetes are associated with insufficient functional beta cell mass, highlighting the urgent need for discovering the mechanisms that regulate beta cell development and expansion. Type 2 diabetes is linked to various cellular stresses that generate supraphysiological levels of reactive oxygen species (ROS). Due to the limited expression of several antioxidant enzymes, beta cells are highly vulnerable to high ROS levels, leading to reduced beta cell proliferation, decreased beta cell identity, diminished insulin content, defective insulin secretion, and enhanced beta cell death, all of which result in the reduction of the functional beta cell mass. Therefore, in order to protect themselves against oxidative stress, beta cells rely on the nuclear factor erythroid 2-related factor (NRF2) antioxidant signaling pathway. Interestingly, several single nucleotide polymorphisms (SNPs) in NRF2 are linked to diabetes.
We recently demonstrated the essential role of NRF2 in the expansion of beta cell mass under metabolic stress and obesity. In that study, published in the journal Diabetes in 2022, we showed that NRF2 is necessary for the adaptive expansion of beta cell mass by reducing oxidative stress, increasing beta cell proliferation, reducing beta cell death, maintaining beta cell identity, and improving glucose homeostasis in high-fat diet (HFD)-fed mice. Importantly, by transplanting pancreatic islets from human cadaveric donors under the mouse kidney capsule, we were able to show that the NRF2 pharmacological activator, bardoxolone methyl, increases both human and mouse beta cell proliferation in vivo. Based on this work, I was awarded a National Institute of Diabetes and Digestive and Kidney Diseases K01 Career Development Award, and two intramural pilot and feasibility grants to characterize the role of NRF2 in the expansion of beta cell mass during embryonic development, neonatal ages, and pregnancy, with the idea that these studies will help to find therapeutic targets that can be leveraged to regenerate beta cells in diabetes.
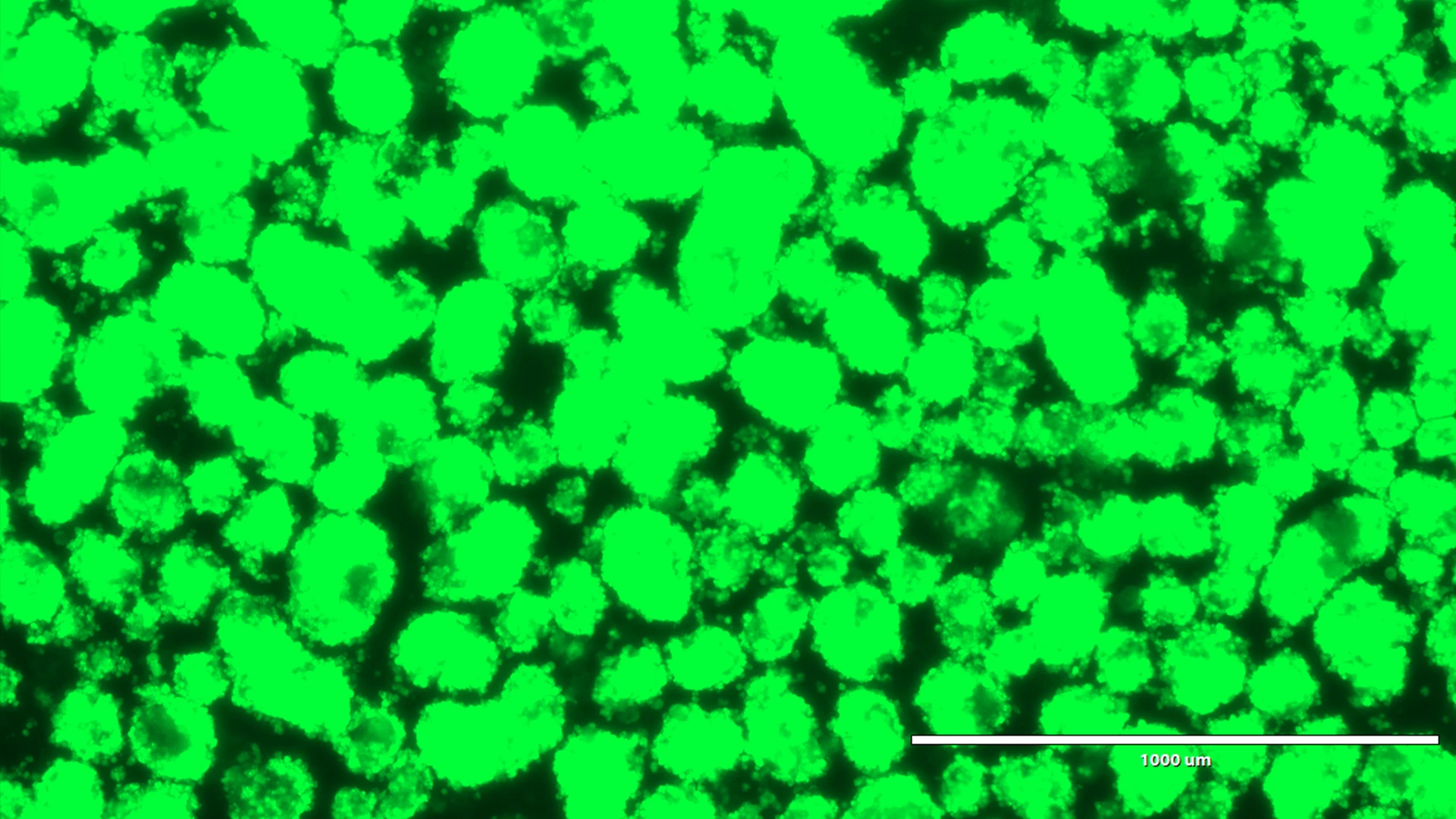
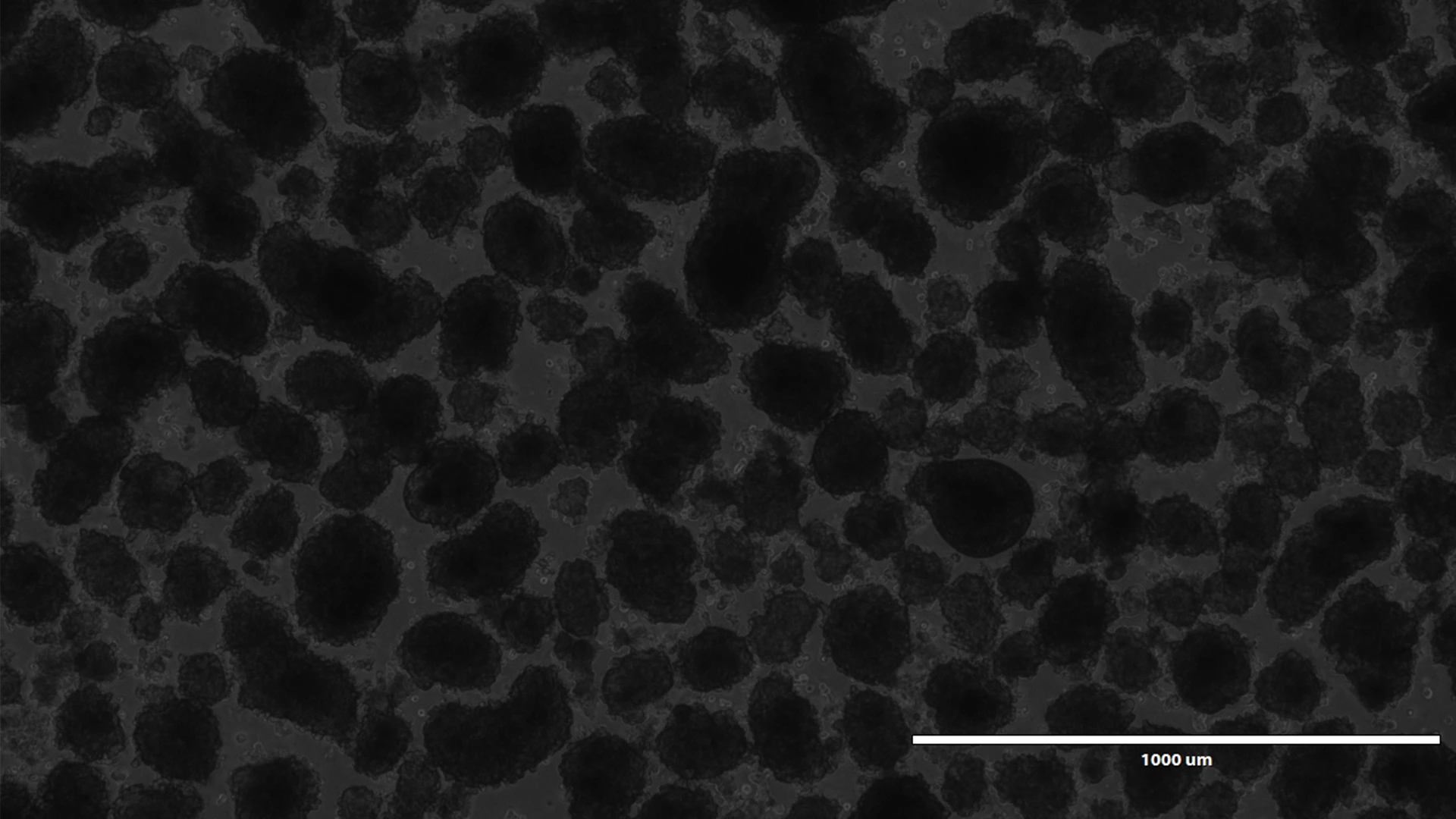
Human pseudoislets five days after lentiviral transduction with a shRNA and a reporter gene under bright light, and blue light.

Romina Bevacqua, PhD
Takeaway Message: Romina Bevacqua, PhD
Proper glucose metabolism in humans crucially depends on acquisition and maintenance of mature physiological functions by pancreatic islets of Langerhans. Failure of pancreatic beta cells to mature increases the risk of, or assures, development of diabetes. Further, our current inability to fully recapitulate this developmental process is a key challenge for islet replacement efforts from renewable sources, such as human stem cell derived beta cells: they yield only immature “beta-like” cells. Understanding the genetic and molecular mechanisms governing the age-dependent development and function of human beta cells is critical to developing better therapeutics for the more than 500 million people with diabetes worldwide. However, while many labs are currently performing in-depth characterization of human islets, very few are able to study human-specific mechanisms in primary, nondividing, adult islet context. Against this background, my lab has developed genetic systems in primary human islet organoids, termed “pseudoislets,” providing unprecedented methods to investigate mechanisms regulating function of mature islets.
These techniques, including specific CRISPR-Cas9 approaches, allow us to activate, silence, or delete genes and noncoding genomic regions in nondividing islet cells from cadaveric donor patients. By deleting human-specific factors and noncoding regulators and examining the effects on islet function, we can begin to understand the roles of these regulators in controlling insulin secretion and complete beta cell maturation. Furthermore, we can also identify novel mechanisms for the regulation and proliferation of beta cells. Our ongoing studies are identifying novel, previously unexplored gene functionalities in islets of Langerhans. We are also introducing novel technological advances to allow site-specific gene editing in primary cells. Altogether, our findings could have translational implications, including identification of novel relevant therapeutic targets for the treatment of an increasing number of people with diabetes.
