Mount Sinai researchers have discovered a family of locally secreted and locally acting molecules in the breast called “mammokines” that contribute not only to normal mammary biology, but to overall fat cell physiology and energy balance control. The research team was led by Prashant Rajbhandari, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease), and a member of the Diabetes, Obesity and Metabolism Institute at the Icahn School of Medicine at Mount Sinai.
The study, which was published in July 2023 in Nature, sheds light on the interplay between mammary adipose tissue and breast health, and suggests possibilities for understanding breast development, lactation, cancer, and obesity and related metabolic disorders.
“The discovery of mammokines is a significant milestone in our quest to comprehend the complex interplay between mammary adipose tissue and breast biology,” says Dr. Rajbhandari. “This breakthrough opens up new avenues for developing targeted interventions to improve breast health and combat related metabolic disorders.”
Mammary adipose tissue has long been recognized for its essential role in breast biology. It consists of many different cell types, including fat cells (adipocytes), immune cells, sympathetic nerve fibers, and mammary epithelial cells forming a milk-producing ductal system. Mammary adipocytes play important roles in organizing mammary ducts, and the reverse is true, as well.
The new study, conducted in a mouse model, reveals an unexpected role for nerve-activated ductal cells in mammary adipocyte metabolism and heat generation. These mammary duct-secreted mammokines play an important role in controlling mammary gland fat abundance and could potentially orchestrate critical processes involved in breast development, lactation, and overall whole-body metabolic regulation. The findings have potential implications for breast cancer, lactation-related disorders, newborn health, and metabolic syndromes linked to mammary adipose dysfunction.
“This breakthrough opens up new avenues for developing targeted interventions to improve breast health and combat related metabolic disorders.”
Prashant Rajbhandari, PhD
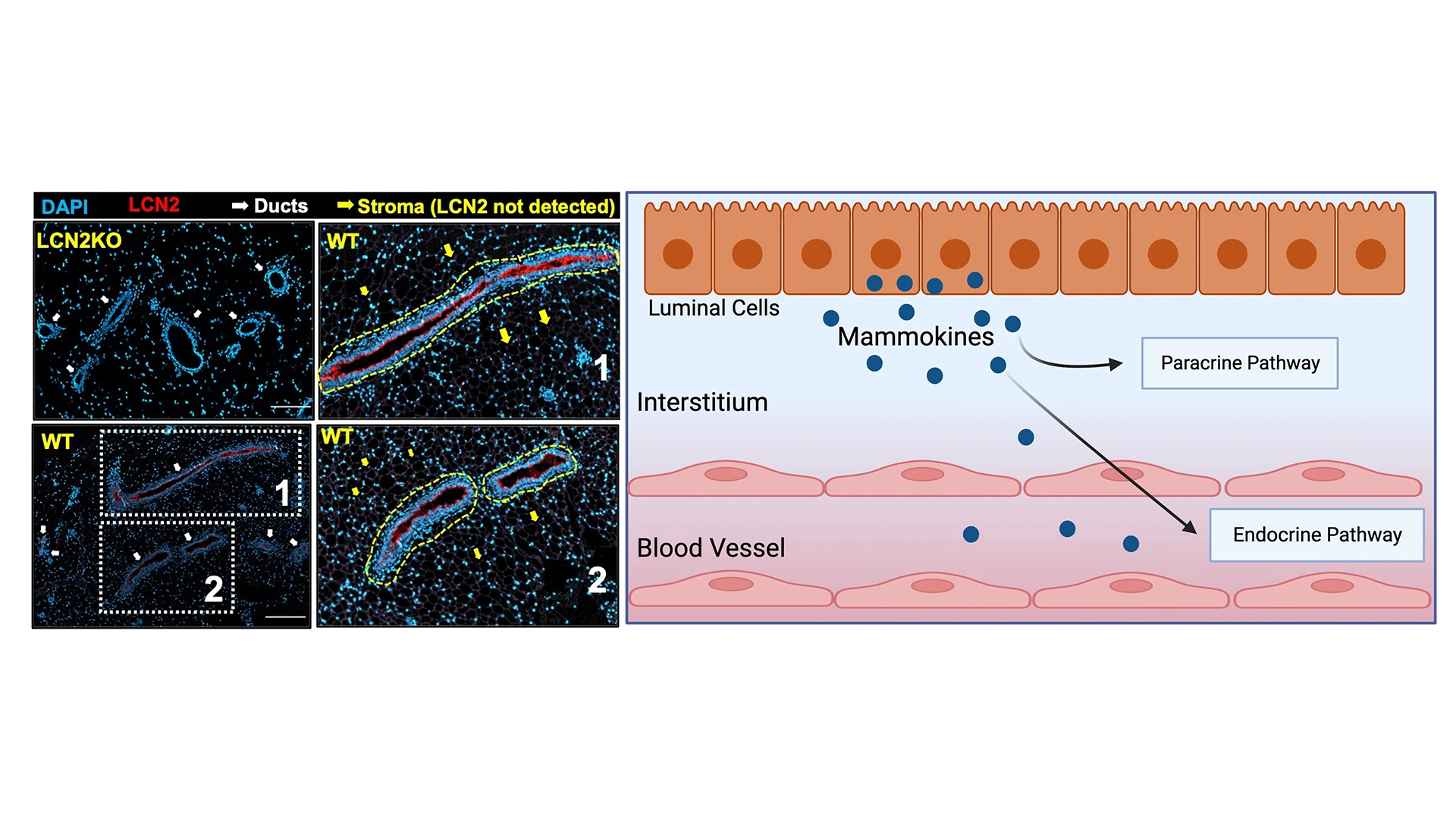
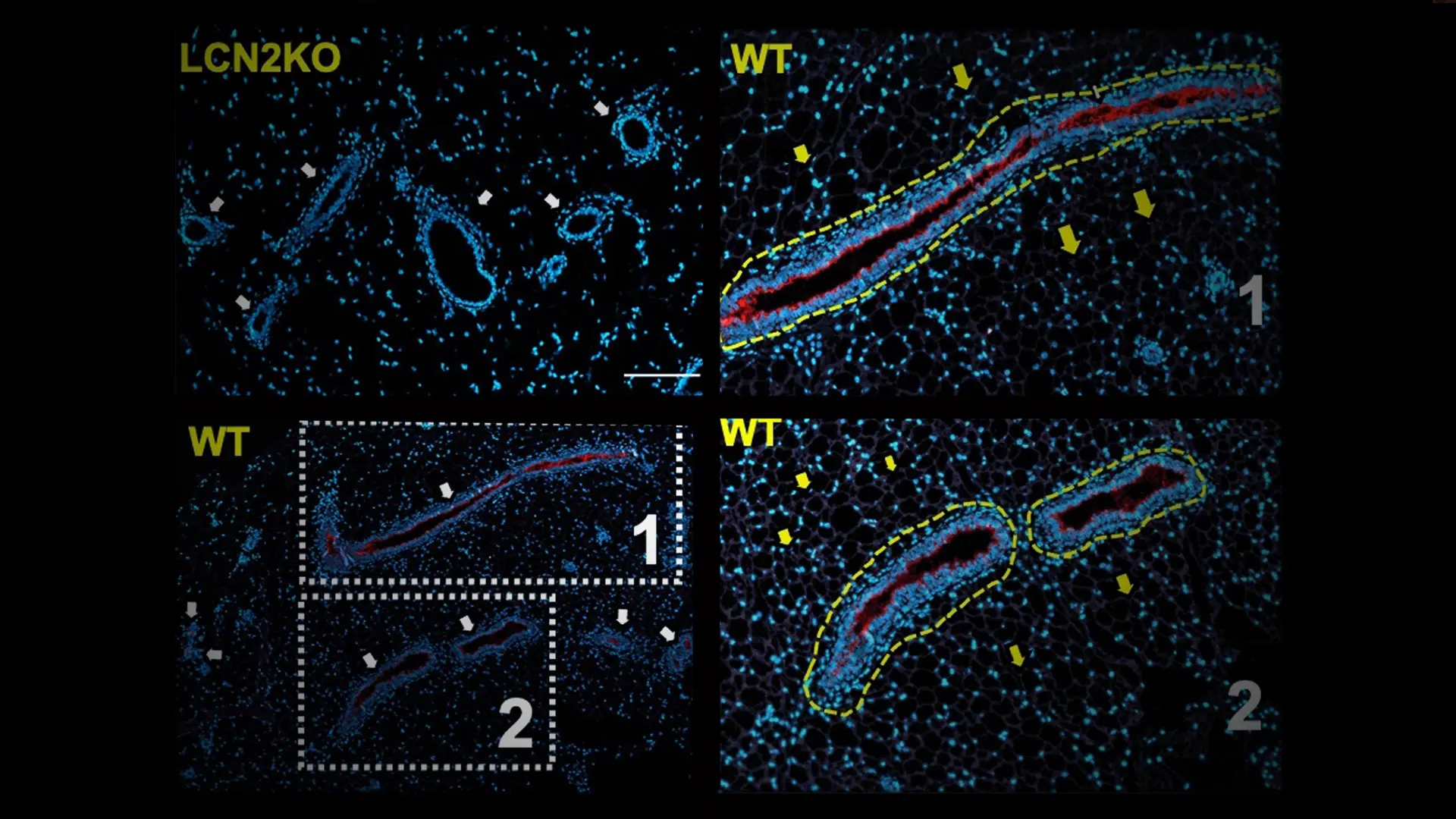
Left: LCN2 is a mammokine involved in maintaining mgWAT adiposity. Immunostaining of mammokine LCN2 and DAPI in the mgWAT of LCN2-KO and wild-type mice. Regions 1 and 2 are enlarged on the right. Dashed yellow outlines indicate LCN2+ ducts. Right: The production of mammokines from luminal epithelial cells, which act on the paracrine pathway in the glandular interstitium and, possibly, on the endocrine pathway via blood vessels.
“Sympathetic activation during cold exposure increases adipocyte thermogenesis via the expression of mitochondrial protein uncoupling protein 1 (UCP1),” the study said. “…Since the mammary duct network extends throughout most of the subcutaneous white adipose tissue in female mice, females show markedly less UCP1 expression, fat oxidation, energy expenditure and subcutaneous fat mass loss compared with male mice, implicating sex-specific roles of mammokines in adipose thermogenesis. These results reveal a role of sympathetic nerve- activated glandular epithelium in adipocyte UCP1 expression and suggest that mammary duct luminal epithelium has an important role in controlling glandular adiposity.”
The research team is now working to further identify new mammokines and characterize each of them. Their primary aim is to decipher the precise roles of these mammokines and determine whether they regulate systemic glucose and insulin homeostasis through communication with the brain, liver, and pancreas. Additionally, the team is exploring potential therapeutic applications for diseases related to the breast and metabolism.
“This work holds enormous promise for future advancements in women's health and metabolic research and the development of personalized treatment strategies based on mammokine profiling,” says Andrew Stewart, MD, Director of the Diabetes, Obesity and Metabolism Institute at Icahn Mount Sinai.
This discovery is the result of the collaborative efforts of a large team of researchers with diverse areas of expertise in the United States and Europe. In addition to Dr. Rajbhandari, research contributors include Sanil Patel, MS; Njeri Z.R. Sparman, BS; Alexandra Alvarsson, PhD; Luís C. Santos, PhD; Samuel J. Duesman, PhD; Chung Hwan Cho, PhD; Ephraim Hathaway, BS; Abha K. Rajbhandari, PhD; Peng Wang, PhD; Leigh Goedeke, PhD; and Sarah A. Stanley, PhD, Icahn Mount Sinai, along with Douglas Arneson, PhD; In Sook Ahn, PhD; Graciel Diamante, PhD; Ingrid Cely, BS; Xia Yang, PhD, and Aldons J. Lusis, PhD, University of California, Los Angeles; Alessia Centonze, PhD, and Cédric Blanpain, PhD, Université Libre de Bruxelles; Noble Kumar Talari, PhD, and Karthickeyan Chella Krishnan, PhD, University of Cincinnati College of Medicine; and Atul J. Butte, PhD, University of California, San Francisco.
Dr. Rajbhandari is supported by the Icahn School of Medicine at Mount Sinai Seed Fund, DK114571, a National Institute of Diabetes and Digestive and Kidney Diseases-supported Einstein-Sinai Diabetes Research Center Pilot and Feasibility Award, and Diabetes Action Research and Education Foundation Grant #501.
Featured

Prashant Rajbhandari, PhD
Assistant Professor, Medicine, Endocrinology, Diabetes and Bone Disease