Castration-resistant prostate cancer, which continues to grow and progresses to lethal disease, even when androgen levels are low or undetectable, presents a major therapeutic challenge with limited therapeutic options, the emergence of treatment resistance, and a poor prognosis.
In encouraging preclinical research, scientists at the Icahn School of Medicine at Mount Sinai have made progress toward understanding, at the molecular and cellular level, how advanced prostate cancer evades treatment, opening new potential avenues to effective therapeutic targeting using novel agents and existing strategies, prior to the prostate tumors becoming castration resistant.
“The goal of our research is to understand the molecular mechanisms and phenotypic dynamic within the tumor microenvironment that drive therapeutic resistance (by impacting cell plasticity and apoptosis among cancer cells) in patients with advanced prostate cancer. Building on that knowledge, our current efforts are invested to develop drugs and define sequencing of treatment strategies to target such changes to overcome resistance and eradicate recurrent disease,” says Natasha Kyprianou, PhD, Professor of Urology, Oncological Sciences, and Pathology, Molecular and Cell Based Medicine at the Icahn School of Medicine and Vice Chair for Research in the Department of Urology.
“The mechanistic insights thus generated from these pre-clinical studies will also provide novel signature tools in the clinical setting for predicting poor clinical outcomes, treatment failure, and patient survival," says Dr. Kyprianou, who is also a member of the Mount Sinai Tisch Cancer Center. The Center has been named a “Comprehensive Cancer Center” by the National Cancer Institute.

“Translational research takes time, but we are excited that these discoveries point toward new ways that we could make a real impact in patients’ lives,” says Natasha Kyprianou, PhD, Professor of Urology, Oncological Sciences, and Pathology, Molecular and Cell Based Medicine at the Icahn School of Medicine and Vice Chair for Research in the Department of Urology. She is also a member of the Mount Sinai Tisch Cancer Center.
The research centers on two molecular processes:
Anoikis (derived from the Greek term for “loss of home”), a form of cell death that normally occurs when cells detach from the extracellular matrix
Epithelial-mesenchymal transition, in which cells undergo a phenotypic transformation from epithelial cells to mesenchymal cells, or vice versa

In the research lab are Campbell Vogt , left, a third-year medical student at the Icahn School of Medicine at Mount Sinai, and Juan Serna, MD, Endourology Fellow-AUA Research Scholar
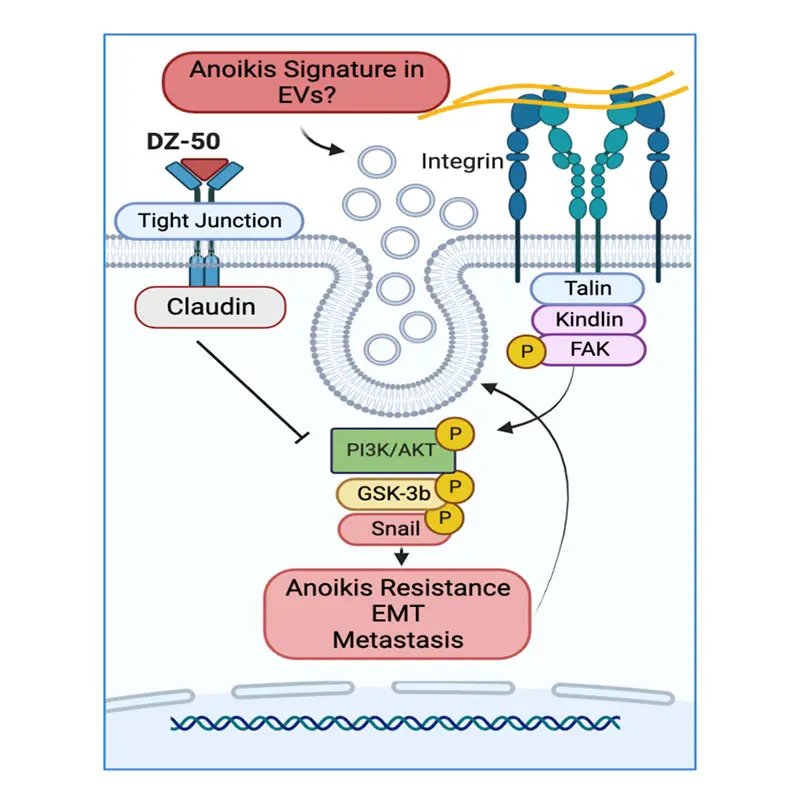
In prior work, Dr. Kyprianou and her colleagues identified anoikis as an important process in advanced prostate cancer and progression to metastasis. “When prostate cancer cells detach from the tumor and become metastatic, they don’t die. If we can target those anoikis-resistant cells, we can stop their metastatic journey,” she says.
Epithelial-mesenchymal transition also drives metastasis. When tumor cells shift from the typical epithelial cells into a mesenchymal state, they become elongated and lose cell-to-cell adhesion. This allows them to become mobile, breaking away from the tumor and entering the bloodstream.
“In the context of metastatic disease, it’s not just a matter of cell survival or cell death. We now understand that the cells can change their phenotype (shape) from epithelial to mesenchymal and back again,” Dr. Kyprianou says. “The combination of anoikis resistance and the epithelial-mesenchymal plasticity confers great advantage to tumor cells that detach from the extracellular matrix, allowing them to become metastatic.”
In research funded by the National Institutes of Health (NIH) and the National Cancer Institute, Dr. Kyprianou explored the intersection of those processes in a study with colleagues, including Navneet Dogra, PhD, Assistant Professor, Pathology, Molecular and Cell Based Medicine, Artificial Intelligence and Human Health, and Ash Tewari, MBBS, MCh, FRCS (Hon.), Dsc (Hon.). Dr. Tewari is Professor and Chair, Milton and Carroll Petrie Department of Urology, Mount Sinai Health System; Chair of Urology, The Mount Sinai Hospital; Director of the Center of Excellence for Prostate Cancer at the Mount Sinai Tisch Cancer Center, Icahn School of Medicine; and Surgeon-in-Chief of the Mount Sinai Tisch Cancer Hospital.
“The question is: How can we exploit those processes at the molecular level so that the cancer cells die by anoikis before they become metastatic?” she says. The research was published in Oncogene in November 2025.
Putting TGF-β to Work
Using human prostate cancer cell line models, the researchers employed RNA sequencing analysis to explore the metabolic changes involved in both anoikis and the epithelial-mesenchymal transition. One key player in the transition is transforming growth factor-β (TGF-β), which has long been recognized for its involvement in cancer. “Prostate cancer cells are known to secrete a lot of TGF-β,” Dr. Kyprianou says.
“In the early stages of prostate cancer, TGF-β suppresses tumors by inducing cell death and blocking proliferation. But if the cancer progresses to metastatic disease, TGF-β becomes a tumor promoter, advancing invasion and migration by inducing the epithelial-mesenchymal transition,” she adds.
In previous work, she and her colleagues developed and patented an experimental compound, DZ50, that induces anoikis. But since mesenchymal cells are naturally resistant to anoikis, the drug is limited in its ability to target metastatic prostate cancer cells, which exist in a mesenchymal state. By harnessing TGF-β to modulate cell plasticity, the team showed it could target cancer cells when they were in an epithelial state and vulnerable to DZ50.
“The conversion between epithelial and mesenchymal phenotypes is a very dynamic process,” Dr. Kyprianou explains. “It’s all about sequencing: First we program the cancer cells to change to an epithelial phenotype so that they are vulnerable to cell death. Then we hit them with the anoikis-inducing drug.”
Translational Research to Halt Advanced Prostate Cancer
Several lines of research are already underway to follow up on the team’s findings. The researchers are hoping to move their anoikis-inducing drug, DZ50, into clinical trials. Meanwhile, they are further exploring the intersection of anoikis, TGF-β, and the epithelial-mesenchymal transition in in-vivo, patient-derived xenograft models.
The researchers are also working to establish biomarkers to identify epithelial and mesenchymal phenotypes of prostate cancer cells. “The goal is to create a liquid biopsy that could identify these markers in patients’ plasma or urine. Then we could predict when a large percentage of cells are in the epithelial phenotype and target them during that window, when they are more vulnerable to cell death,” Dr. Kyprianou says. Such a biomarker could also be useful for predicting therapeutic response to treatment and identifying patients at high risk of metastasis, she adds.
“Translational research takes time, but we are excited that these discoveries point toward new ways that we could make a real impact in patients’ lives,” Dr. Kyprianou says.

The research team, from left: Sara Darbandi, PhD; Campbell Vogt; Eduard Reznik, MD, PhD; Natasha Kyprianou, MBBS, PhD; Juan Serna, MD; Lauren Martires; and Maria Kouspou, PhD.
As part of the work on castration-resistant prostate cancer (CRPC), Dr. Kyprianou and leading investigators Navneet Dogra, PhD, and Ash Tewari, MBBS, MCh, FRCS (Hon.), Dsc (Hon.), recently received an R01 grant from the NIH’s National Cancer Institute.
One of the key objectives of the grant is establishing predictive signatures of lymph node invasion and tumor microenvironment (TME) prognostication in prostate cancer patients.
The team hypothesizes that intercellular communications between prostate tumor cells and lymph nodes can lead to invasion and metastasis, and extracellular vesicles (EVs) act as extracellular conveyors to facilitate lymph node involvement. The team will pursue pathological assessment of the phenotypic landscape of primary prostate tumors, CRPC and lymph node metastasis, and molecular profiling of EVs in blood specimens to determine their contribution to lymph node invasion in prostate cancer patients undergoing prostatectomy.
The goal is to establish the correlation between differential profiles of lymph node invasion and phenotypic effectors in prostate tumors and in EVs from prostate cancer patients during clinical progression to advanced disease.
This work will lead to the development of noninvasive tools for predicting prostate tumor progression and lymph node invasion and targeted therapeutic strategies to overcome tumor recurrence. The proposed studies will lay a foundation for the use of anoikis-inducing agents to target the TME and to treat metastatic CRPC and clinically relevant signatures predictive of invasive properties in patients with advanced prostate cancer (affecting clinical decision making).