In the quest to achieve improved outcomes among patients who present with muscle-invasive bladder cancer, Mount Sinai researchers are looking beyond conventional radiographic tools to the potential of circulating tumor DNA (ctDNA) assays to detect micro-metastatic disease.
Led by John Sfakianos, MD, Mount Sinai is assessing the efficacy of Signatera™, a highly sensitive molecular residual DNA (MRD) test. The test is unique in that it uses patient tumor DNA to find traces of the disease circulating in the bloodstream.
“This is a newer test that has gained traction in other diseases such as colorectal cancer,” says Dr. Sfakianos, Professor of Urology and Urologic Oncology at the Icahn School of Medicine at Mount Sinai. “What we are finding through our studies is that it can be used for everything from assisting in the initial diagnosis of cancer to monitoring how well patients respond to treatments.”
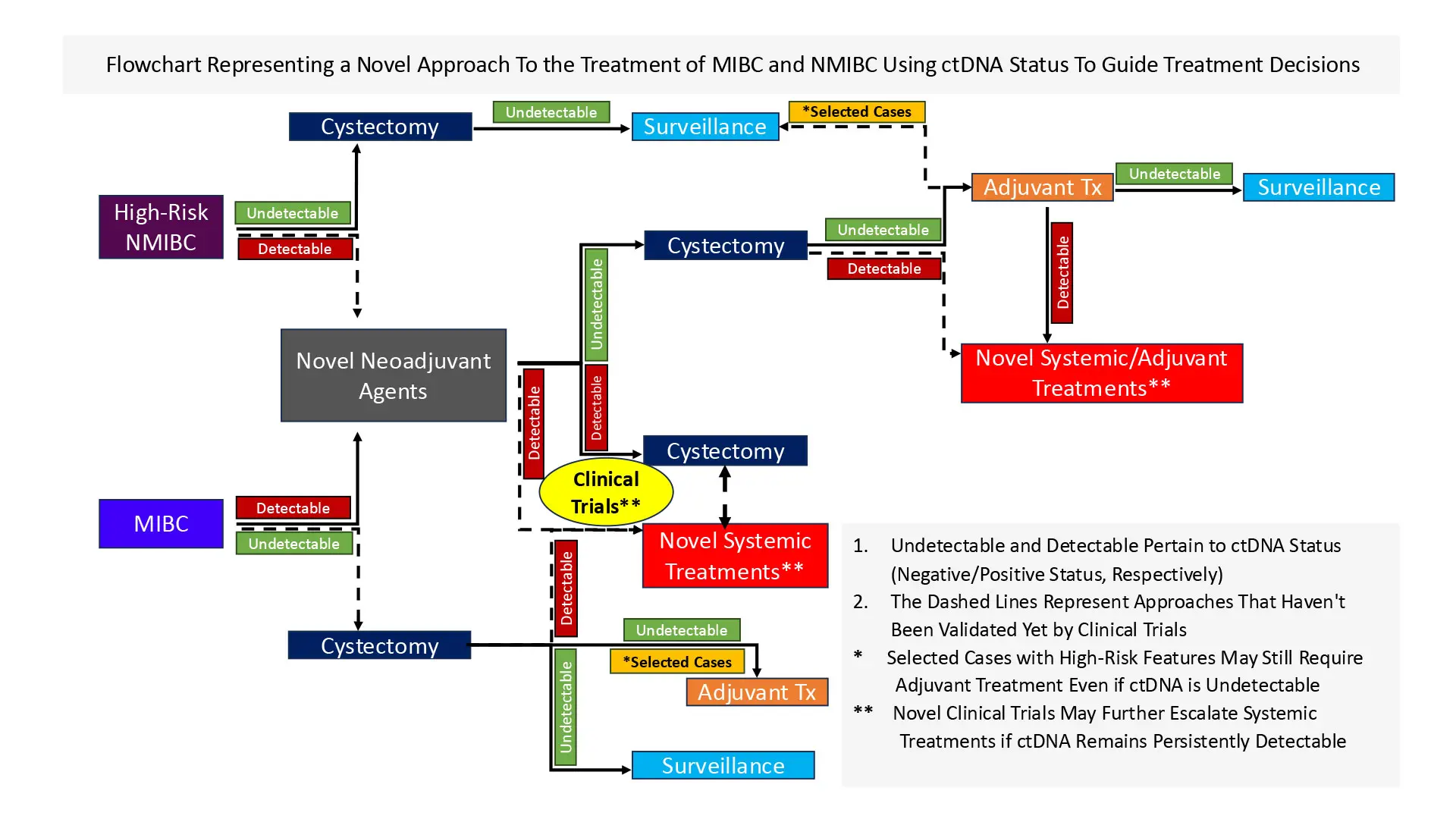
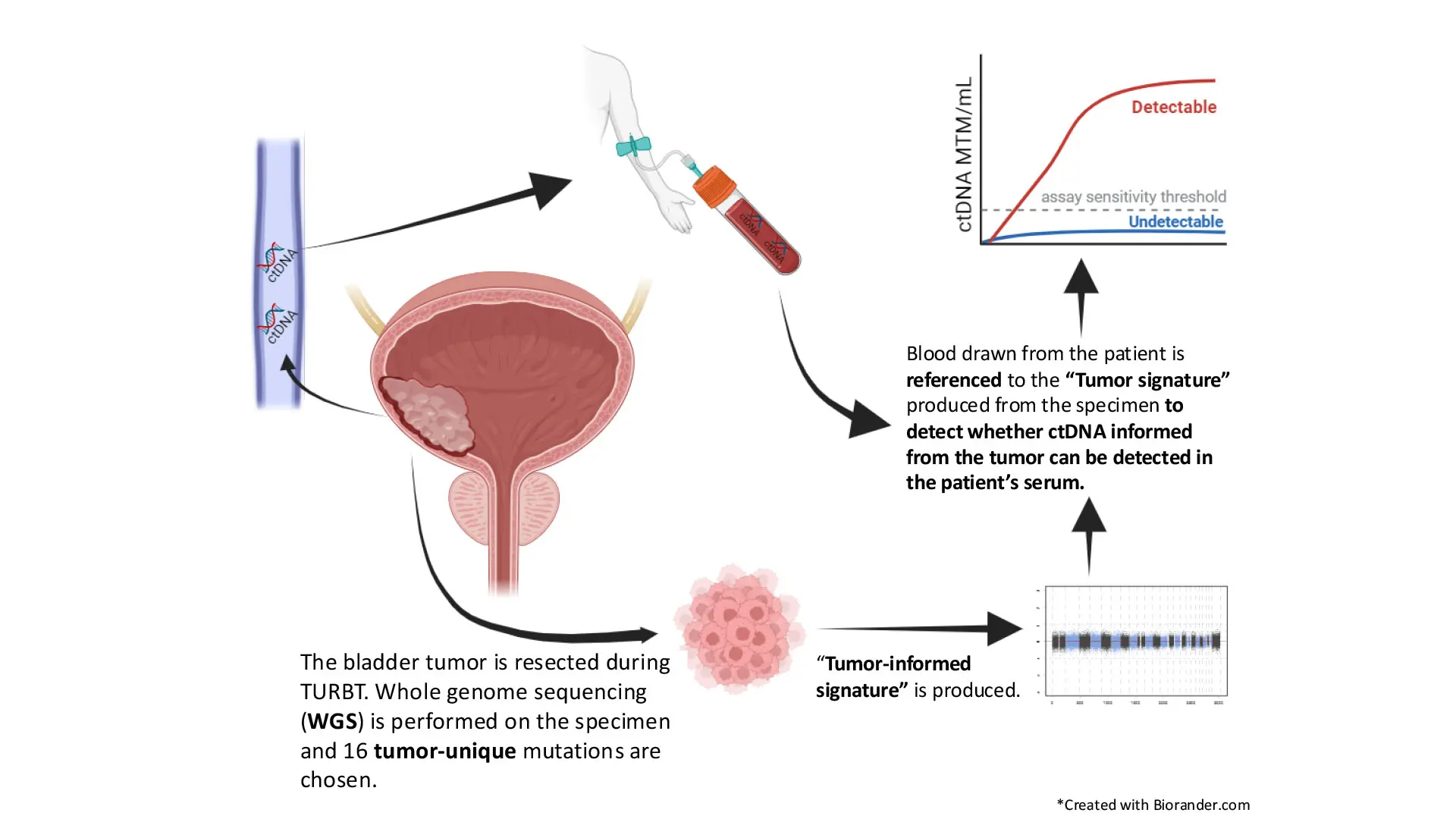
Dr. Sfakianos’s findings are based on two years of data gleaned from using the assay among his patients and retrospective studies he has led and on which he has collaborated. In one multicenter investigation, he and a team of researchers assessed the prognostic and predictive value of Signatera among 167 patients, using 852 plasma samples collected before radical cystectomy (RC) and during MRD and surveillance windows. The study, “Association of Tumor-informed Circulating Tumor DNA Detectability Before and After Radical Cystectomy with Disease-free Survival in Patients with Bladder Cancer,” was published in July 2024 in European Urology Oncology.
The team found that detectable ctDNA during MRD and surveillance windows was associated with shorter disease-free survival (DFS) rates when compared to undetectable ctDNA (MRD: hazard ratio 6.93; p < 0.001; surveillance: hazard ratio 23.02; p < 0.001). They also noted that patients with undetectable ctDNA did not appear to benefit from adjuvant therapy (p = 0.34). Detectable ctDNA in the pre-RC (p = 0.045), MRD (p = 0.002), and surveillance (p < 0.001) windows was the only risk factor independently associated with shorter DFS.
“Our findings suggest that ctDNA testing among patients with bladder cancer undergoing radical cystectomy is prognostic and potentially predictive, and that identifying patients with a high risk for recurrence may enhance patient counseling and decision-making,” Dr. Sfakianos says.
In a second study, Dr. Sfakianos and a team of researchers assessed whether pre-RC ctDNA status is predictive of pathological and oncological outcomes and changes in status post-RC in relation to recurrence-free survival (RFS). The study, “Longitudinal Tumor-informed Circulating Tumor DNA Status Predicts Disease Upstaging and Poor Prognosis for Patients Undergoing Radical Cystectomy,” was published in October 2024 in European Urology Oncology.
Among the 112 participants chosen for the study, 59 (53 percent) had detectable pre-RC ctDNA, which was associated with poor RFS (log-rank p < 0.0001). Pre-RC detectable ctDNA was associated with poor outcomes regardless of clinical stage (<cT2 vs ≥cT2) and neoadjuvant therapy. Multivariable analyses further revealed that pre-RC detectable ctDNA was associated with a higher risk of nodal disease (odds ratio 5.4, 95 percent confidence interval [CI] 1.9-18.2; p = 0.003) and locally advanced disease (odds ratio 3.6, 95 percent CI 1.5-9; p = 0.005). Cox regression analyses showed that pre-RC detectable ctDNA (hazard ratio 4.5, 95 percent CI 1-19; p = 0.04) and detectable MRD ctDNA (hazard ratio 9.9, 95 percent CI 2.6-37; p < 0.001) were predictive of disease recurrence.

John Sfakianos, MD, Professor of Urology and Urologic Oncology at the Icahn School of Medicine at Mount Sinai
“The fact that patients who are undetectable for ctDNA do fine with surgery alone whereas those who have detectable levels have the worst outcomes regardless of treatment is the key finding for us,” Dr. Sfakianos says. “Regardless, we need to do better in helping patients who are detectable.”
Based on these studies, Dr. Sfakianos is proposing a study to assess the safety of surgery only in patients where ctDNA is undetectable. The goal now, he says, is to conduct further validation of this promising biomarker through a prospective patient study.
“I believe that is what we need to do to establish whether this can be a gold standard for making patient treatment decisions with confidence,” he says.