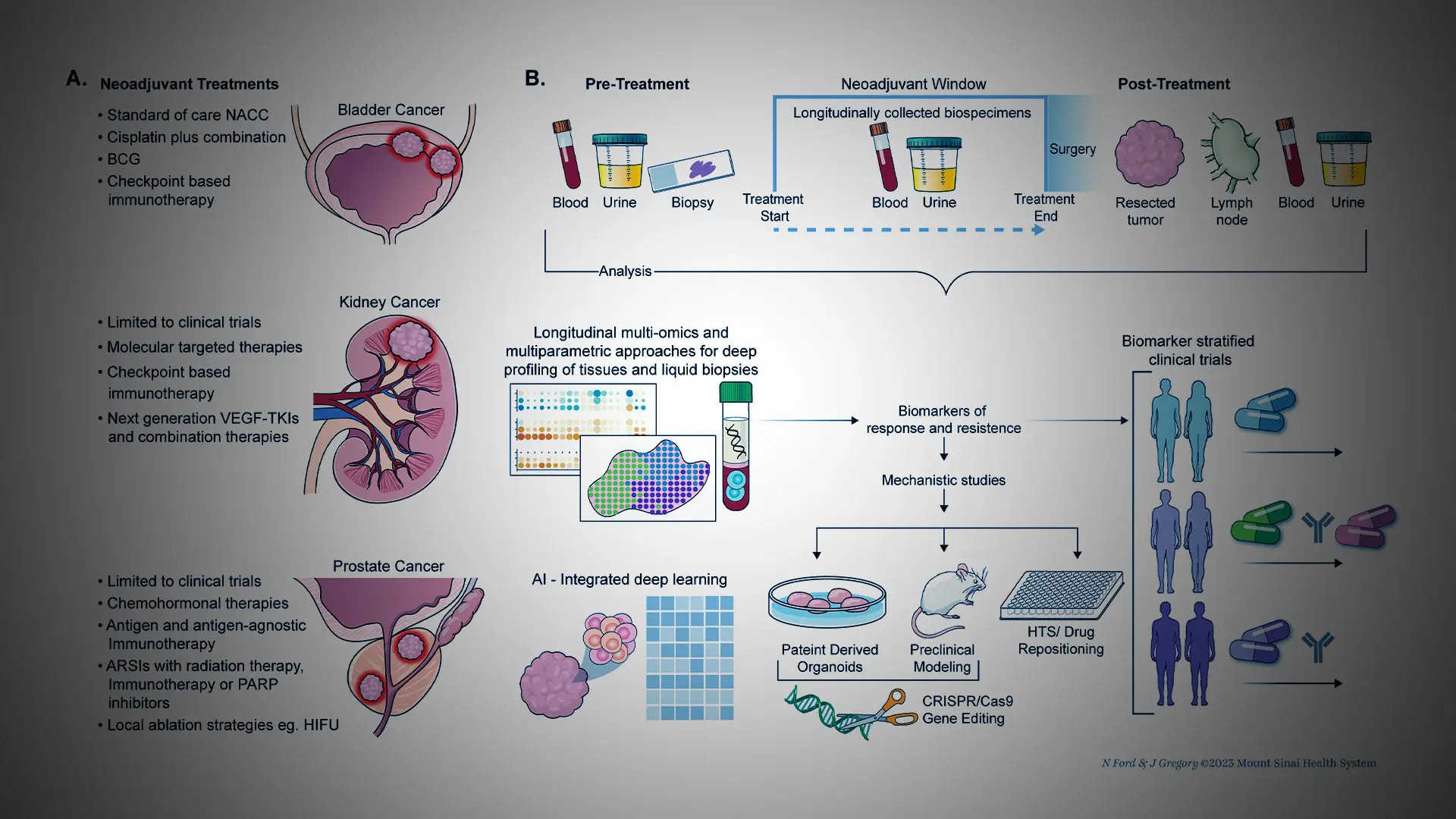
While many bladder cancer patients have benefited from neoadjuvants—medical therapies delivered before surgery—their application in prostate and kidney cancers is currently confined to clinical trials.
With an interest in extending these improved outcomes to other cancers of the urinary and male reproductive organs, researchers at the Icahn School of Medicine at Mount Sinai investigated related neoadjuvant studies and how they might inform future study designs to benefit more patients. The paper, titled “Genitourinary (GU) cancer neoadjuvant therapies: Current and future approaches,” was published online September 6, 2023, in Trends in Cancer.
“We believe that our work provides the most up-to-date information on neoadjuvant approaches for these cancers. Neoadjuvant therapies can make treatment easier, shrink tumors for surgery, and help predict subsequent treatment response,” says first and corresponding author Sujit S. Nair, PhD, Assistant Professor of Urology and Director of Genitourinary Immunotherapy Research at Icahn Mount Sinai. “There is much interest in the field for expanding the benefits of preoperative cancer therapies to reduce recurrence and boost survival in patients with local or locally advanced genitourinary cancers and address this important unmet need.”
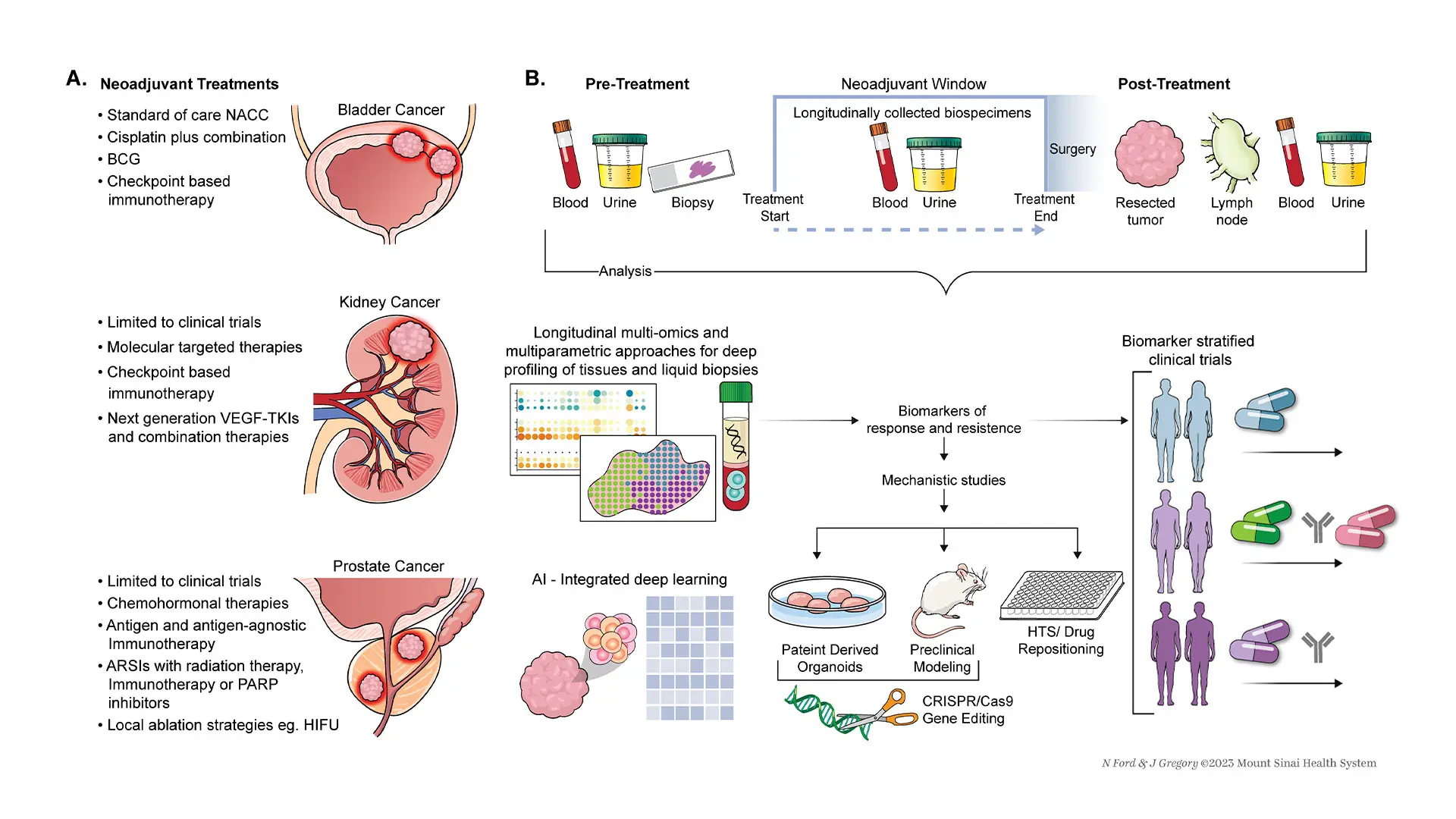
Researchers at the Icahn School of Medicine at Mount Sinai explored whether the success seen in bladder cancer with neoadjuvant therapies might inform future study designs to benefit more patients, including those with kidney and prostate cancer.
While neoadjuvant therapies have been a standard treatment for bladder cancer, the researchers hope that changes are imminent to extend the benefits to patients with prostate and kidney cancers.
This approach is clinically relevant since it targets primarily previously untreated individuals, potentially maximizing drug response, say the investigators. In addition, the outcomes remain unaffected by other anticancer treatments.
“Importantly, in neoadjuvant trials, predictive biomarkers can guide patient selection of those likely to respond best. Neoadjuvant trials have the potential to greatly enhance the development of novel drugs and combination therapies,” says Nina Bhardwaj, MD, PhD, co-corresponding author and Director of Immunotherapy, Co-Director of the Cancer Immunology Program, and Professor of Medicine (Hematology and Medical Oncology) at The Tisch Cancer Institute at Mount Sinai.
Led by senior and co-corresponding author Ash Tewari, MBBS, MCh, FRCS (Hon.), Chair of Urology of the Mount Sinai Health System and Surgeon-in-Chief of the Tisch Cancer Hospital, the research team has significant expertise in genitourinary cancer clinical trials, particularly in the neoadjuvant setting.
“Apart from this work, we’ve recently completed a study of a neoadjuvant in prostate cancer patients at high risk. In addition, more studies that match patients based on biomarkers to test novel treatment combinations are expected to begin in early 2024. We’re aiming to work closely with the Food and Drug Administration early on to determine the right benchmarks of success for the study to potentially accelerate approval,” says Dr. Tewari.
The other authors of the paper are Dimple Chakravarty, PhD, DVM, and Vaibhav Patel, MD.
Featured

Ash Tewari, MBBS, MCh, FRCS (Hon.)
Chair of Urology of the Mount Sinai Health System and Surgeon-in-Chief of the Tisch Cancer Hospital

Nina Bhardwaj, MD, PhD
Ward Coleman Chair in Cancer Research

Sujit S. Nair, PhD
Assistant Professor of Urology and Director of Genitourinary Immunotherapy Research