For more than 40 years, an inactivated form of tuberculosis bacterium known as Bacillus Calmette-Guerin (BCG) has been the only first-line therapy for urologists treating non-muscle invasive bladder cancer (NMIBC). The clinical results–about half of all patients develop recurring tumors following treatment with BCG–have underscored the need for novel therapeutic agents for a disease that is diagnosed each year in more than 181,000 new patients worldwide.
A team of clinician-researchers from Mount Sinai’s Department of Urology has dedicated itself to that search, buoyed by three new grants that are allowing members to actively explore a unique working model of BCG resistance based on a combination immune checkpoint blockade strategy targeting NKG2A natural killer (NK) cells and PD-L1 expression on recurring NMIBC tumors. One of those grants, $2.5 million from the Bladder Cancer Advocacy Network (BCAN), is paving the way for a phase 2 clinical trial expected to begin enrolling by mid-2024.
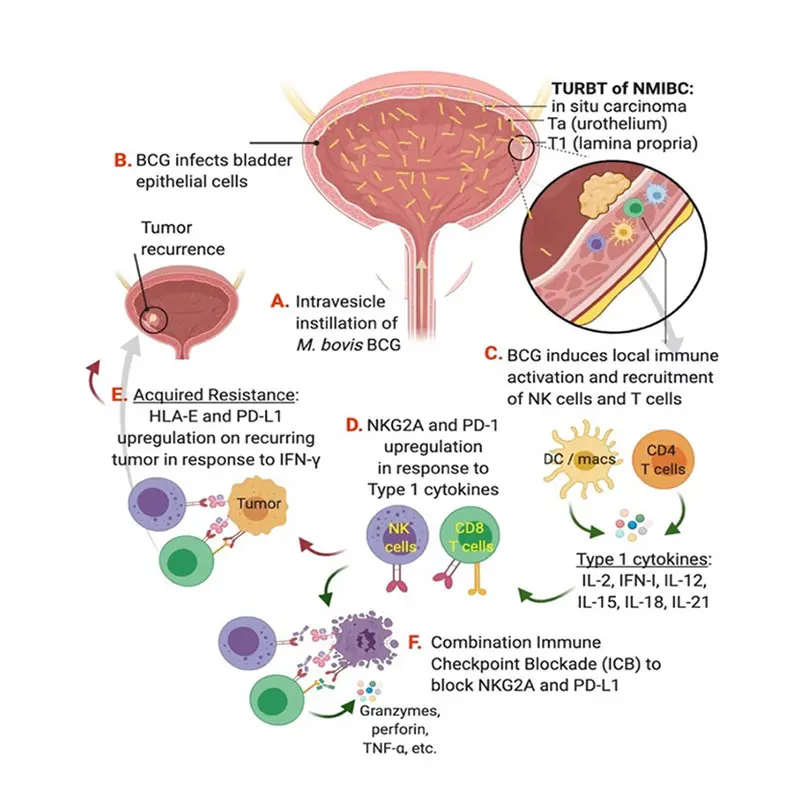
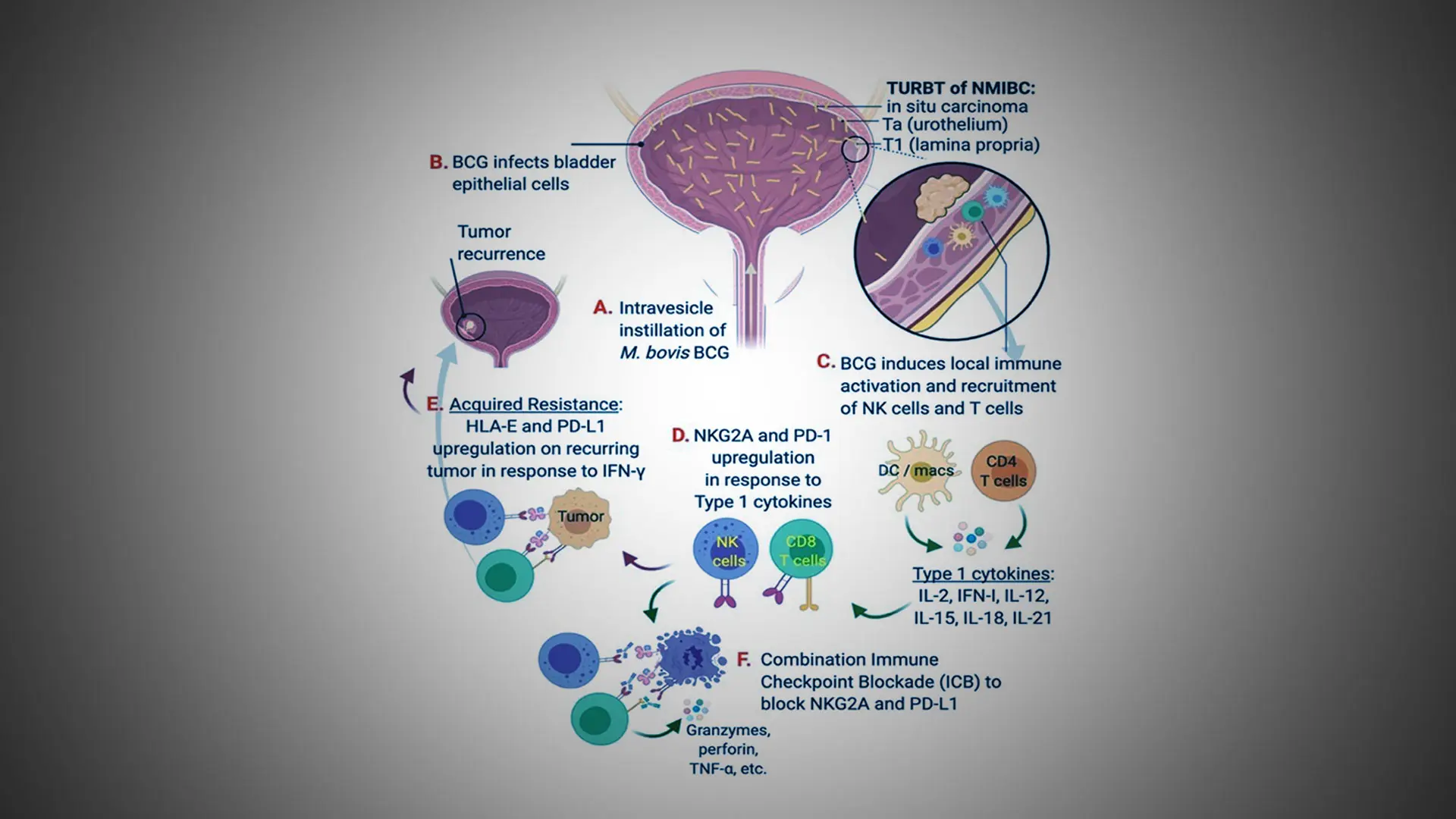
Model of BCG resistance and strategy for combination immune checkpoint blockade targeting NKG2A and PD-L1.
“When BCG doesn’t work for patients, we have very few options other than surgery to remove their bladder,” says John Sfakianos, MD, Associate Professor of Urology and Urologic Oncology at the Icahn School of Medicine at Mount Sinai, who is leading the research along with colleague Amir Horowitz, PhD, Associate Professor, Oncological Sciences. “Our theory is that chronic activation from repeat exposure to BCG drives immune dysregulation in a significant number of patients. That’s why the thrust of our research for the past five years has been looking at blood, tissue, and urine samples from patients before and after treatment with BCG to identify the source of that resistance within the immune system.”
The field has not been without progress. In 2020, the monotherapy PD-1 inhibitor pembrolizumab became the first treatment to be approved by the U.S. Food and Drug Administration for refractory NMIBC in more than 20 years. Its durable response rate, however, is only around 20 percent.
“The inability to improve on BCG therapy and develop strong new options is due largely to a lack of understanding of the stromal environment and tumor cell interactions,” says Dr. Horowitz, internationally known for his work in the field of NK cell immunology. “We’ve made important inroads in our lab by showing that normally functioning NK cells and CD8 T cells, which are essential for anti-cancer immunity at all stages of bladder cancer, are often impaired in the tumor microenvironment due to their dysregulation or exhaustion.”
The team’s working model is carefully exploring that mechanistic thread. It has found, for example, that increased Interferon-gamma (IFN-y) signaling in BCG-unresponsive NMIBC correlates with the upregulation of both PD-L1 and human leukocyte antigen (HLA)-E, a major histocompatibility complex class I molecule expressed at low levels on hematopoietic cells that can be induced by inflammatory signals. Just as important, Mount Sinai researchers and others have identified HLA-E and its receptor, NKG2A, as a unique checkpoint axis driving resistance to BCG therapy–and thus an attractive target for inhibition.

“Our hope and expectation is these projects will lead to clinical trials—similar to the one we’re about to launch—and our findings will translate readily from clinic to bedside," says John Sfakianos, MD, Associate Professor of Urology and Urologic Oncology at the Icahn School of Medicine at Mount Sinai, who is leading the research along with colleague Amir Horowitz, PhD.
“This pathway suggests to us that in vitro blockade of NKG2A, either alone or in combination with PD-L1 blockade, stimulates NK function and a strong CD8 T cell response to HLA-E/PD-L1 expression on recurring tumors,” says Dr. Sfakianos, whose clinical focus is on treating patients with urothelial cancers of the upper urinary tract and the bladder.
In addition to the grant fueling the upcoming phase 2 clinical trial, the Bladder Cancer Advocacy Network is funding two other studies by Dr. Sfakianos. One is a $2.5 million R01 grant that will use high-resolution molecular imaging to examine BCG resistance within the context of the immune system landscape. The second study is focused more on identifying unique resistance mechanisms within the NMIBC tumor itself.
“These projects recognize the hard work we’ve done in the field of non-muscle invasive bladder cancer, and we believe they’ll continue to improve our understanding of BCG and, potentially, other novel therapeutic approaches,” says Dr. Sfakianos. “Our hope and expectation is these projects will lead to clinical trials—similar to the one we’re about to launch—and our findings will translate readily from clinic to bedside.”