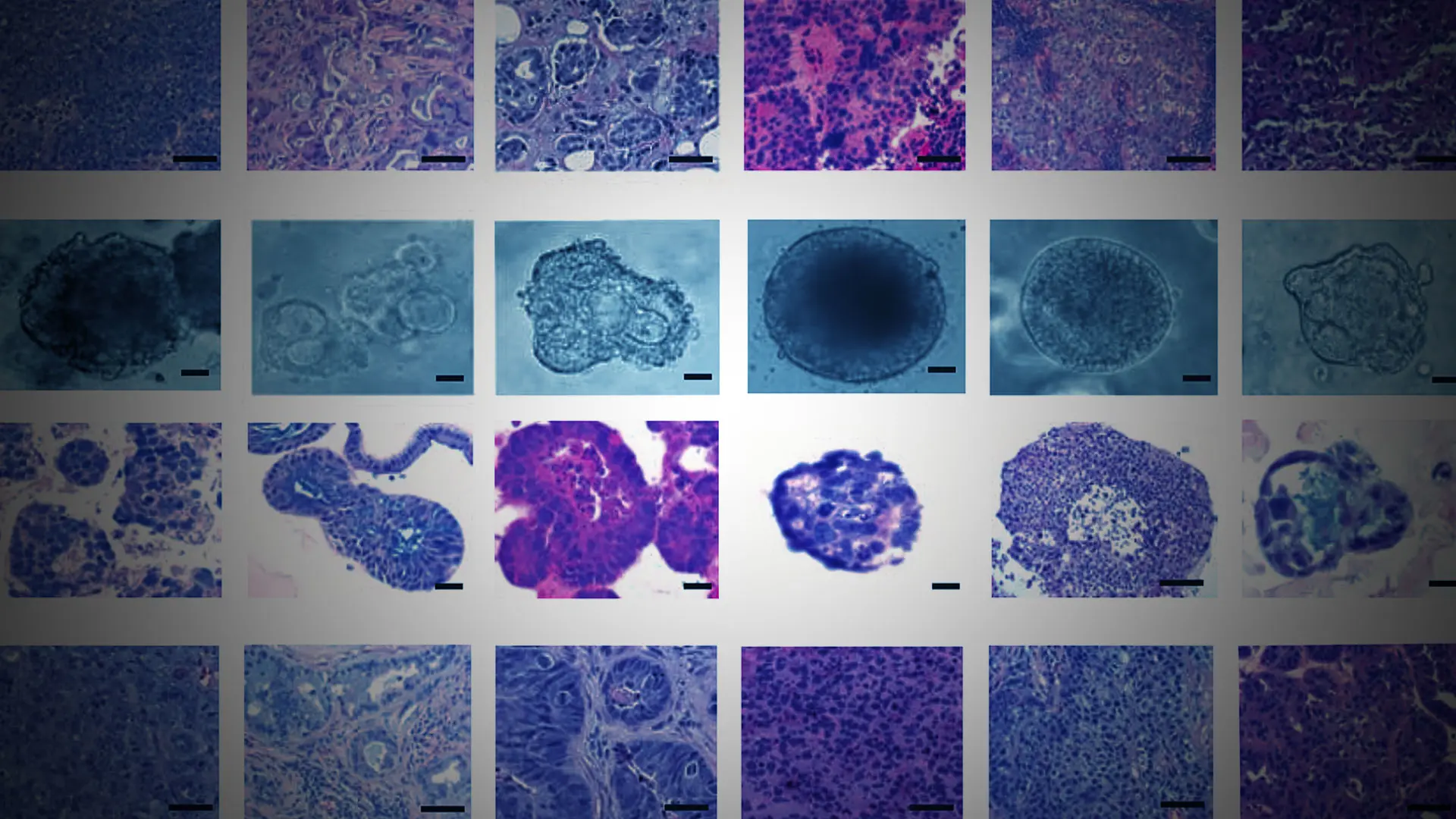
Advances in imaging, biomarkers, and genetic testing continue to benefit patients who present with prostate and bladder cancers. But patients with kidney cancers have mostly been left behind due to a general lack of research funding from federal and industry sources.
An initiative developed by Ketan K. Badani, MD, could help close that gap by generating new diagnostic tools and insights on risk factors and optimizing treatment for kidney cancer patients.
Supported by philanthropic donations, Dr. Badani launched a kidney cancer research pipeline in 2016 to identify characteristics associated with aggressive and benign tumors to enable more informed clinical decision-making on cases requiring therapeutic intervention.
“Similar to prostate cancer, some kidney tumors progress very slowly and are not fatal while others are aggressive and are fatal,” says Dr. Badani, Director of the Kidney Cancer Center at the Mount Sinai Health System, and Professor of Urology at the Icahn School of Medicine at Mount Sinai. “We do not have sophisticated tools to discern which ones will follow an aggressive path. This is a significant question for kidney cancer, and one we hope to answer through this research.”
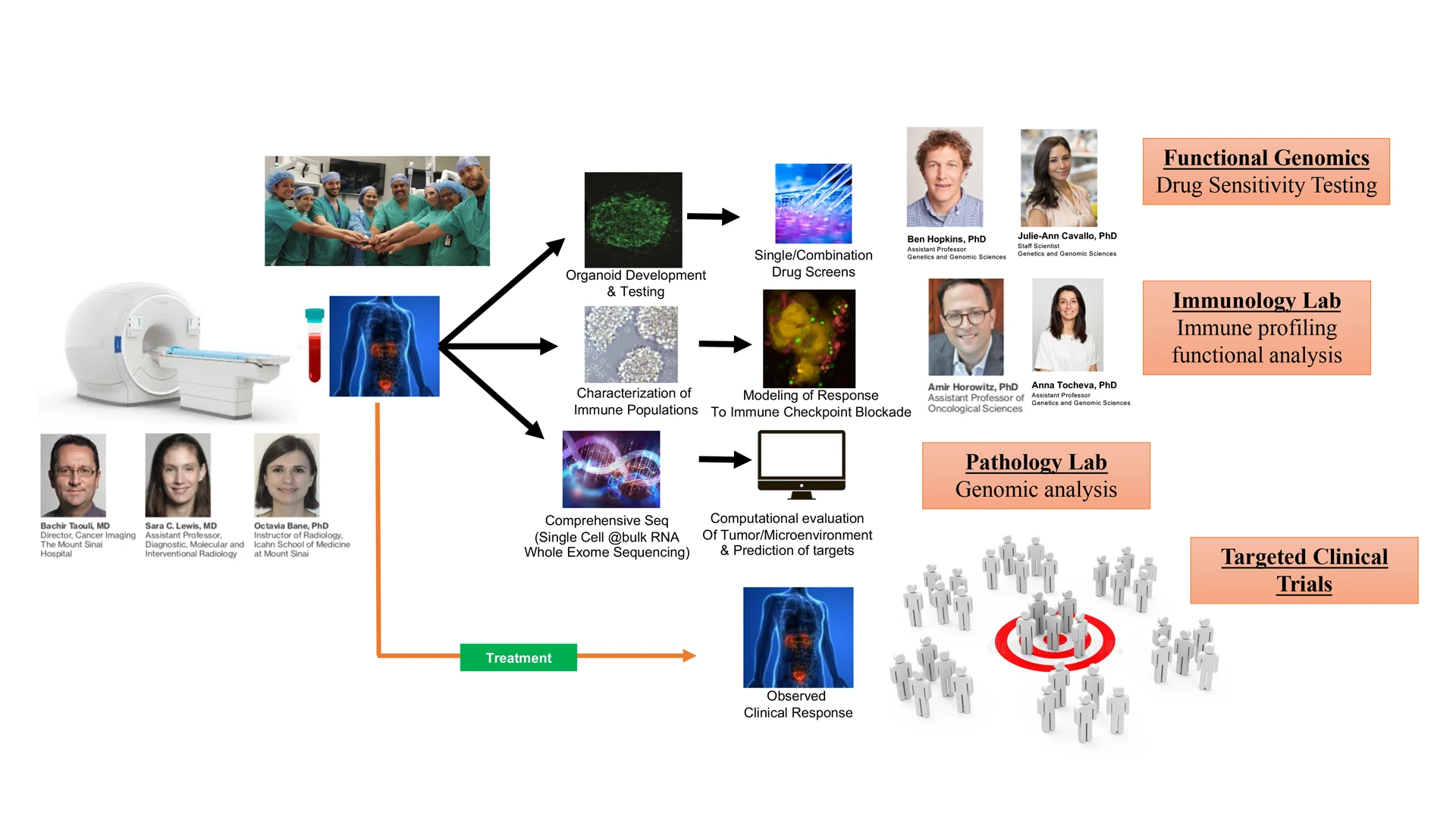
Patients who present with a tumor requiring a robotic partial nephrectomy are offered an opportunity to enroll in clinical trials being conducted at Mount Sinai. Prior to surgery, they undergo multiparametric magnetic resonance imaging (MRI) to identify tumor features that are associated with an aggressive pathology. Post-surgery, the patient’s tumor is assessed by a pathologist, as well as research scientists at The Tisch Cancer Institute, and those findings are correlated with the results of the multiparametric MRI.
Tumor, blood, and urine samples collected from consented patients are entered into Mount Sinai’s BioMe® Biobank Program, an electronic medical record-linked initiative that facilitates genetic, epidemiologic, molecular, and genomic studies. Tumor samples are also provided to the Marc and Jennifer Lipschultz Precision Immunology Institute, which conducts studies of the tumor microenvironment to assess aggressiveness, and to Benjamin D. Hopkins, PhD, Assistant Professor of Genetics and Genomic Sciences, and Oncological Sciences, at Icahn Mount Sinai, and co-leader of the Functional Genomics Pipeline at The Tisch Cancer Institute, who uses them to grow organoids for therapeutic agent studies.
“To my knowledge, there is no other center nationwide that has established a pipeline like this with a seamless process for harvesting kidney tissue and liquid samples for assessment and research through multiparametric MRI, genetic immunology, and organoids,” says Dr. Badani. “We have the scientists and resources in place that enable us to do all of this on-site, which is unique in itself and positions us to make significant advances in this field.”

Ketan K. Badani, MD, Director of the Kidney Cancer Center at the Mount Sinai Health System, and Professor of Urology at the Icahn School of Medicine at Mount Sinai
Each scientist and laboratory participating in the pipeline has its own research protocols that determine which patients they engage. Decisions are typically made during bi-weekly meetings in which Dr. Badani and his research team present patient cases scheduled for robotic kidney surgery.
“There may be a case in which Dr. Hopkins opts not to grow an organoid as he has several matches for that specific tumor, but the patient will be selected to undergo a multiparametric MRI,” Dr. Badani says. “Ideally, we would like to have every patient included at every stage because that would give them access to a wealth of useful genetic sequencing, tumor microenvironment, and organoid information about their tumor.”
Several key insights on kidney disease characteristics and pathology have come from patients who have participated in the research. For example, in a study involving 104 renal cell carcinomas and 21 benign lesions from 125 patients, Dr. Badani and his colleagues found that machine learning models that incorporate MRI-based radiomics features and qualitative radiologic assessment can help characterize renal masses. Meanwhile, immunology researchers identified four biomarkers using proteomic and transcriptomic data that distinguish severity of prognosis in clear cell renal cell carcinoma (ccRCC). They also found that these biomarkers can be used to predict two-year and five-year overall survival in ccRCC patients across different tumor stages. Once validated, these findings will provide invaluable information to guide intervention and surveillance decisions among patients.
Based on advances such as these, Dr. Badani is confident the research pipeline he has created could enable more precise filtering of patients for surveillance and robotic partial nephrectomy within the next five years, which, in turn, could significantly improve patient outcomes.
“The prognosis for kidney cancer is either really good or really bad,” he says. “Through this research pipeline, we have the opportunity to change the way we treat the disease by eliminating overtreatment of indolent tumors because we will know with certainty which ones are lethal.”