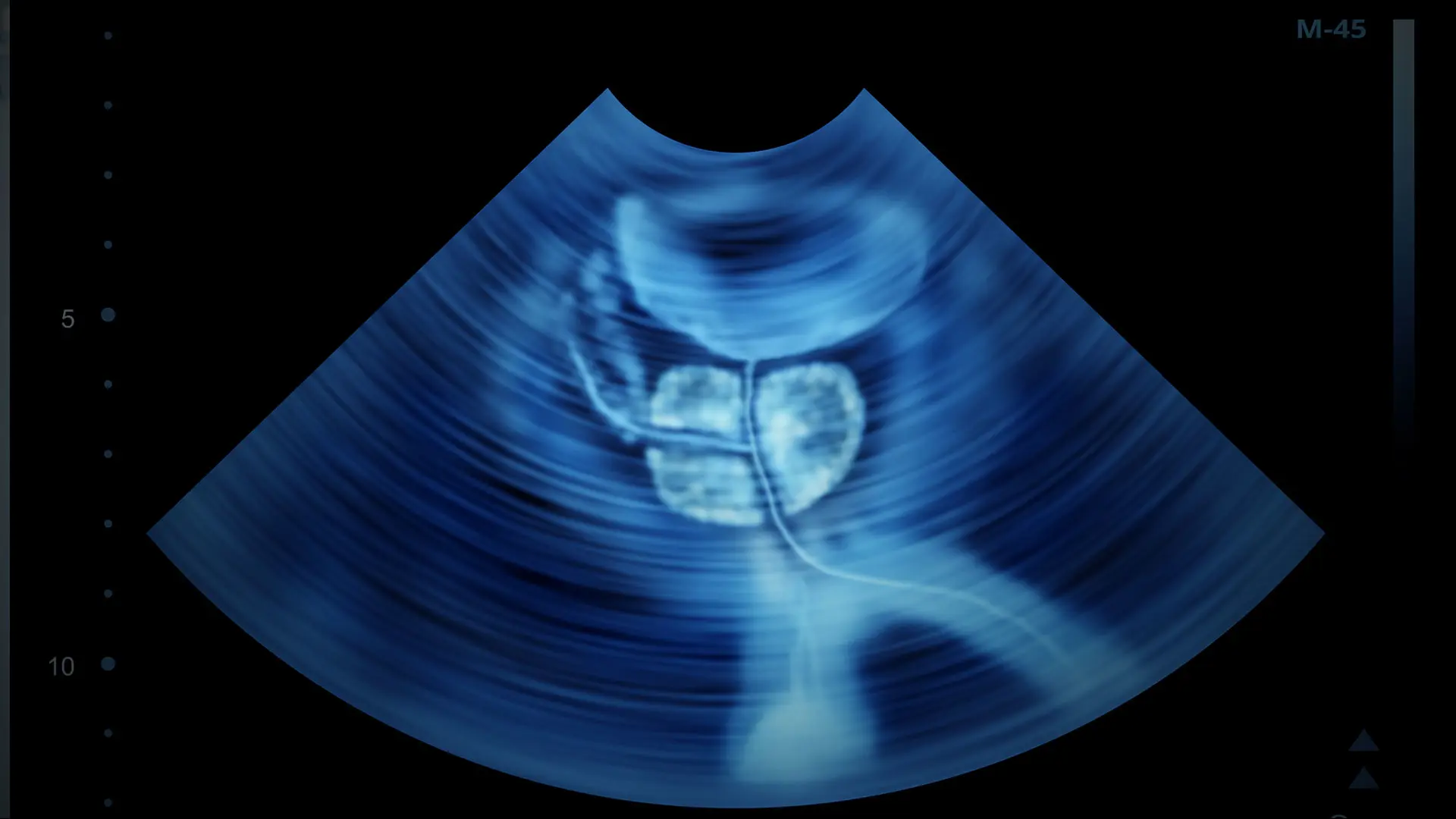
Mount Sinai has begun offering patients high-intensity focused ultrasound (HIFU) for targeted ablation of localized prostate tumors, the latest in a series of innovations to improve men’s health and another option for those seeking a minimally invasive approach.
HIFU fuses high-resolution imaging and biopsy data with real-time ultrasound imaging, which provides integrated, detailed 3D views of the prostate, enabling doctors to deliver high intensity sound waves that allow the ablation of prostate tissue with minimal morbidity.
The procedure greatly reduces the risk of side effects, including urinary incontinence and erectile dysfunction, often associated with radical prostatectomy and radiation. The procedure is performed on an outpatient basis.
“Studies in Europe and preliminary studies in the United States point to the effectiveness of HIFU,” says Avinash Reddy, MD, Assistant Professor, Department of Urology, Icahn School of Medicine at Mount Sinai. “However, longer follow-up is still needed to truly accept HIFU as a standard of care for men with localized prostate cancer. Nevertheless, the arrival of this treatment is significant because of the degree of precision with which the cancer can be treated in the prostate, sparing healthy tissue and reducing side effects.” The treatment option was first approved by the U.S. Food and Drug Administration in 2015, with approvals for devices from manufacturers coming in following years.
Mount Sinai recommends HIFU for patients with localized prostate cancer (stage T1/T2) whose Gleason score is less than or equal to 7 and whose prostate specific antigen (PSA) is less than or equal to 15. HIFU is also a better and feasible alternative for patients who are not candidates for surgery because of their general condition, such as those with morbid obesity or cardiovascular disease. HIFU is only feasible in patients with a maximum prostate volume of 80 ml. If the prostate volume is greater than 80 ml, the size of the prostate will be reduced by endoscopic resection two months before HIFU treatment to remove the entire adenomatous part of the gland, or institute neoadjuvant hormonal treatment for three to six months.
In earlier iterations, HIFU resulted in hemiablation or unilateral partial ablation. With this newest technology, high-resolution images fused with biopsy results and real-time ultrasound imaging provide images in 3D that enable doctors to draw precise contours around the diseased tissue and ablate only that portion of the prostate, minimizing damage to surrounding tissue.
Instead of using radiation, high-intensity sound waves are used to heat up and burn the targeted tissue, choking off the blood supply and causing cell death. The magnitude of ultrasound energy delivered rapidly raises the temperature at the focal point, causing coagulation necrosis without damaging healthy tissue and structures outside the targeted area. The procedure is repeatable and can be followed by external radiotherapy.
Studies in Europe and preliminary studies in the United States have demonstrated the safety and efficacy of the procedure on more than 1,000 patients. But there are no long-term follow-up data in the United States. Urologic surgeons in the United States generally need 10 years of data to establish focal therapy as a standard treatment.
“We are comfortable with the data and will be monitoring closely and building on our experience,” says Dr. Reddy.
More than 65,000 men around the world with localized prostate cancer have gone through the HIFU procedure. Since the first-generation HIFU system was introduced in France, European researchers have published many peer-reviewed studies of the use of HIFU for whole gland ablation.
In 2013, The Journal of Urology published a German study of 704 patients treated with HIFU, in which co-investigators concluded that long-term follow-up with HIFU therapy demonstrated a high overall rate of cancer-specific survival (99 percent) and 10-year salvage treatment-free rates of 98 percent in low-risk (meaning these patients did not require additional treatment for cancer after 10 years), 72 percent in intermediate-risk, and 68 percent in high-risk patients, very comparable to other standard treatments.
In the United States, Houston Methodist Hospital showed HIFU to be safe and effective, with good oncologic control for prostate cancer patients. The study was formally presented at a sectional meeting of the American Urological Association in 2018. In this study, clinicians evaluated clinical outcomes of 24 HIFU patients between July 2016 and July 2018. All patients received whole gland ablation and 80 percent of patients had PSA scores below 0.5 ng/ml.
HIFU is partially covered by Medicare, and is covered by many insurance companies as a salvage treatment if the patient’s cancer returns after going through radical prostatectomy or radiation therapy. However, the American Medical Association has begun the process of establishing a Category 1 CPT code for HIFU. This will make it possible for men with localized prostate cancer who have the HIFU procedure to be reimbursed by their health insurers, starting January 1, 2021.
“Our Department has long been a leader in pioneering the adoption of novel diagnostic techniques, and this is another example of how we are continuing to expand our program to offer patients a range of innovative treatment options,” says Dr. Reddy.
Featured

Vivek Reddy, MD
Director of Cardiac Electrophysiology, and the Leona and Harry B. Helmsley Charitable Trust Professor of Cardiac Electrophysiology