The Department of Urology at the Icahn School of Medicine at Mount Sinai is building on its legacy of innovation in research and therapeutic treatments with two new National Institutes of Health grants focused on the mechanisms that drive renal cell carcinoma and erectile dysfunction among males who undergo radical prostatectomy.
For the renal cell carcinoma study, Babu Padanilam, PhD, Professor and Director of Kidney Research in the Department of Urology at the Icahn School of Medicine, and Ketan Badani, MD, Professor of Urology and Vice Chair of Urology and Robotic Operations at the Mount Sinai Health System, will be exploring the role of renal nerves and nerve-derived factors in the initiation and progression of renal cell carcinoma in both denervated and wild type animal models. The research is rooted in previous studies conducted by Dr. Padanilam, which found that denervation prevented development of kidney fibrosis.
“We are going to inject cancer cells into the renal capsule or renal parenchyma in denervated kidneys, and assess whether the absence of the nerve impacts renal carcinoma progression,” says Dr. Padanilam, who joined Mount Sinai in January 2021 from the College of Medicine at the University of Nebraska Medical Center. “If we find there is no progression, we can start to look at the factors that the nerves are secreting that lead to decreased or absent progression, which could provide a target for therapeutic interventions.”
In the second study, Dr. Padanilam is partnering with Ash Tewari, MBBS, MCh, Professor and Chair, Milton and Carroll Petrie Department of Urology, and Kyung Hyun Kim, MD System Chair in Urology, to look at mechanisms of nerve generation and causation of erectile dysfunction following robotic-assisted radical prostatectomy.
“Despite advances in nerve-sparing techniques, approximately 40 percent of men who undergo radical prostatectomy experience sexual dysfunction,” says Dr. Padanilam. “The risk of complications is such that many men opt to live with the cancer instead. That is a major concern for us, so we are using animal models and cell cultures to look at the role of nerves and specific nerves involved, to see how this damage is occurring, and determine how we can improve the surgical techniques to prevent nerve damage, regenerate the damaged nerves to minimize or even prevent erectile dysfunction.”
Current therapeutic measures for nerve injuries are nerve guidance conduits or a nerve graft, but Dr. Padanilam indicates they are not effectively promoting regeneration. He believes the immune response that occurs post-surgery may be a more viable treatment target.
“There is some evidence of crosstalk occurring between immune cells and nerve cells that may impact nerve regrowth,” he explains. “I am interested in whether the immune cells are secreting agents that are preventing the nerves from regrowing or if there are some growth-promoting agents that can enhance nerve regeneration.”
Dr. Padanilam will also be looking at the role of migrating neuronal cells in erectile dysfunction as well as in development of prostate cancer. Recent research has suggested that nerve cells may be migrating to the prostate tumor through the blood and contributing to tumor initiation and growth.
“What we do not know is what is causing this migration to occur,” he says. “Is this migration occurring due to normal factors or are there certain cancer-related factors that are driving this migration? Our goal is to look at the factors that drive that migration, see if there is increased migration after prostate cancer initiation, and assess whether the cancer is causing the nerve cells to secrete factors that could be promoting tumor growth. If we can identify those factors, we can potentially block them and prevent progression of the prostate cancer.”
In addition to these studies, Dr. Padanilam is continuing his research on the molecular mechanisms that result in cellular injury in acute kidney injury (AKI) and renal fibrogenesis in chronic kidney disease (CKD).
For AKI, he is looking at metabolic changes that inhibit glycolysis and fatty acid oxidization—the two main mechanisms by which adenosine triphosphate (ATP) is synthesized in the proximal tubules. He has identified the activation of Poly [ADP-ribose] polymerase 1 (ARP-1) and p53 as factors in inhibiting glycolysis and the activation of p53 and cyclophilin D as factors in inhibiting fatty acid oxidation.
“The hypothesis is that activation of these molecules in the renal proximal tubules after AKI limits their capacity to generate ATP, thus leading to cell death due to energy depletion,” Dr. Padanilam says. “We found that blocking p53 or cyclophilin D in animal models was protective against kidney injury, restoring fatty acid oxidation to normal levels, and thus the proximal tubules’ capacity to produce ATP. The goal is to explore this observation further in clinical trials, and I am working with pharmaceutical scientists to develop therapeutics that can target these specific molecules in the proximal tubules.”
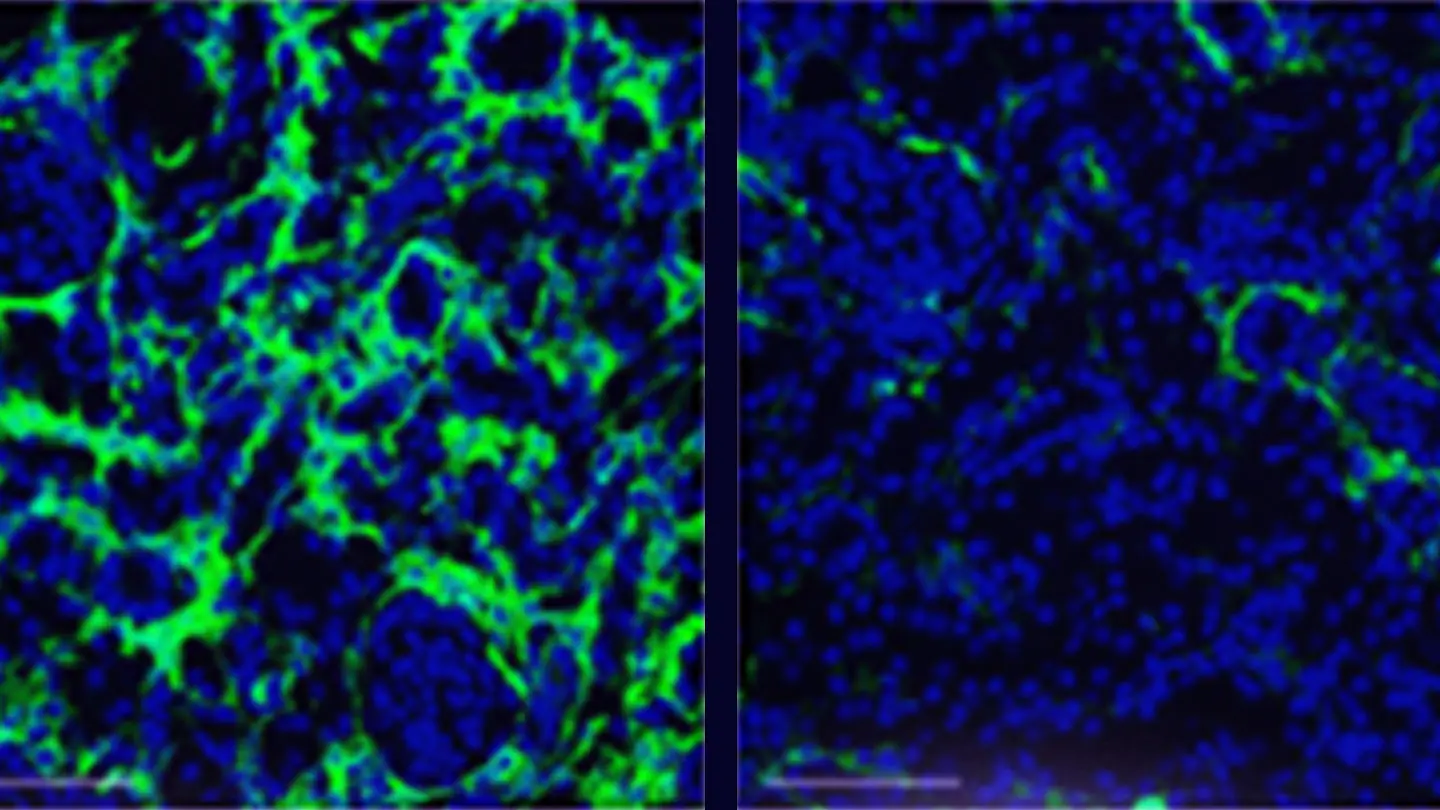
For CKD, Dr. Padanilam is expanding his initial observation that renal denervation prevents development of renal fibrosis. He is looking at the mechanisms by which the nerve-derived norepinephrine signaling via the alpha 2 adrenergic receptor (α2-AR) induces fibrosis and assessing whether inhibition of α2-AR subtypes or its signaling pathways has therapeutic value in preventing renal fibrogenesis.
“We identified nerve-derived norepinephrine as the driving factor of renal fibrogenesis, signaling via its α2-AR subtype receptors in animal models of CKD,” Dr. Padanilam says. “There are Food and Drug Administration-approved therapeutics for α2-AR, and the plan is to repurpose these drugs and conduct clinical trials to assess the effectiveness of blocking this receptor and thus preventing renal fibrogenesis.”
Ultimately, Dr. Padanilam hopes his efforts to identify the metabolic factors involved in cellular injury and nerve regeneration will have wide-ranging impacts for patients who have kidney, liver, and/or prostate disease.
“If we can find the mechanisms that are driving cancer, energy depletion, or nerve regeneration, we can identify therapeutic agents, optimize the conditions, dosage, and route of administration in animal models, and take that into clinical trials,” he says. “If we are successful, we can make a difference in the lives of our patients.”
40%
of men who undergo radical prostatectomy experience sexual dysfunction
“Despite advances in nerve-sparing techniques, approximately 40 percent of men who undergo radical prostatectomy experience sexual dysfunction,” says Dr. Padanilam. “The risk of complications is such that many men opt to live with the cancer instead. That is a major concern for us, so we are using animal models and cell cultures to look at the role of nerves and specific nerves involved, to see how this damage is occurring, and determine how we can improve the surgical techniques to prevent nerve damage, regenerate the damaged nerves to minimize or even prevent erectile dysfunction.”
Current therapeutic measures for nerve injuries are nerve guidance conduits or a nerve graft, but Dr. Padanilam indicates they are not effectively promoting regeneration. He believes the immune response that occurs post-surgery may be a more viable treatment target.
“There is some evidence of crosstalk occurring between immune cells and nerve cells that may impact nerve regrowth,” he explains. “I am interested in whether the immune cells are secreting agents that are preventing the nerves from regrowing or if there are some growth-promoting agents that can enhance nerve regeneration.”
Dr. Padanilam will also be looking at the role of migrating neuronal cells in erectile dysfunction as well as in development of prostate cancer. Recent research has suggested that nerve cells may be migrating to the prostate tumor through the blood and contributing to tumor initiation and growth.
“What we do not know is what is causing this migration to occur,” he says. “Is this migration occurring due to normal factors or are there certain cancer-related factors that are driving this migration? Our goal is to look at the factors that drive that migration, see if there is increased migration after prostate cancer initiation, and assess whether the cancer is causing the nerve cells to secrete factors that could be promoting tumor growth. If we can identify those factors, we can potentially block them and prevent progression of the prostate cancer.”
In addition to these studies, Dr. Padanilam is continuing his research on the molecular mechanisms that result in cellular injury in acute kidney injury (AKI) and renal fibrogenesis in chronic kidney disease (CKD).
For AKI, he is looking at metabolic changes that inhibit glycolysis and fatty acid oxidization—the two main mechanisms by which adenosine triphosphate (ATP) is synthesized in the proximal tubules. He has identified the activation of Poly [ADP-ribose] polymerase 1 (ARP-1) and p53 as factors in inhibiting glycolysis and the activation of p53 and cyclophilin D as factors in inhibiting fatty acid oxidation.
“The hypothesis is that activation of these molecules in the renal proximal tubules after AKI limits their capacity to generate ATP, thus leading to cell death due to energy depletion,” Dr. Padanilam says. “We found that blocking p53 or cyclophilin D in animal models was protective against kidney injury, restoring fatty acid oxidation to normal levels, and thus the proximal tubules’ capacity to produce ATP. The goal is to explore this observation further in clinical trials, and I am working with pharmaceutical scientists to develop therapeutics that can target these specific molecules in the proximal tubules.”
For CKD, Dr. Padanilam is expanding his initial observation that renal denervation prevents development of renal fibrosis. He is looking at the mechanisms by which the nerve-derived norepinephrine signaling via the alpha 2 adrenergic receptor (α2-AR) induces fibrosis and assessing whether inhibition of α2-AR subtypes or its signaling pathways has therapeutic value in preventing renal fibrogenesis.
“We identified nerve-derived norepinephrine as the driving factor of renal fibrogenesis, signaling via its α2-AR subtype receptors in animal models of CKD,” Dr. Padanilam says. “There are Food and Drug Administration-approved therapeutics for α2-AR, and the plan is to repurpose these drugs and conduct clinical trials to assess the effectiveness of blocking this receptor and thus preventing renal fibrogenesis.”
Ultimately, Dr. Padanilam hopes his efforts to identify the metabolic factors involved in cellular injury and nerve regeneration will have wide-ranging impacts for patients who have kidney, liver, and/or prostate disease.
“If we can find the mechanisms that are driving cancer, energy depletion, or nerve regeneration, we can identify therapeutic agents, optimize the conditions, dosage, and route of administration in animal models, and take that into clinical trials,” he says. “If we are successful, we can make a difference in the lives of our patients.”
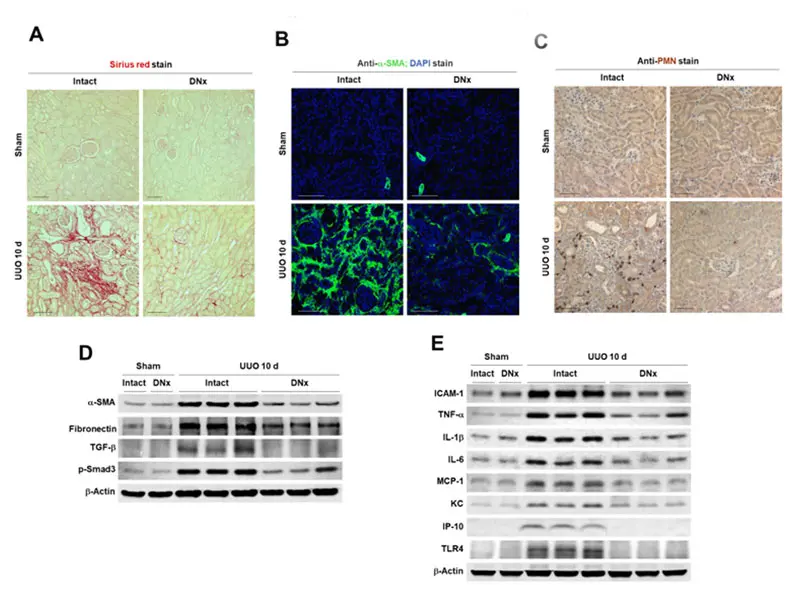
Renal denervation prevents tubulointestitial fibrogenesis and inflammation during chronic kidney disease progression.
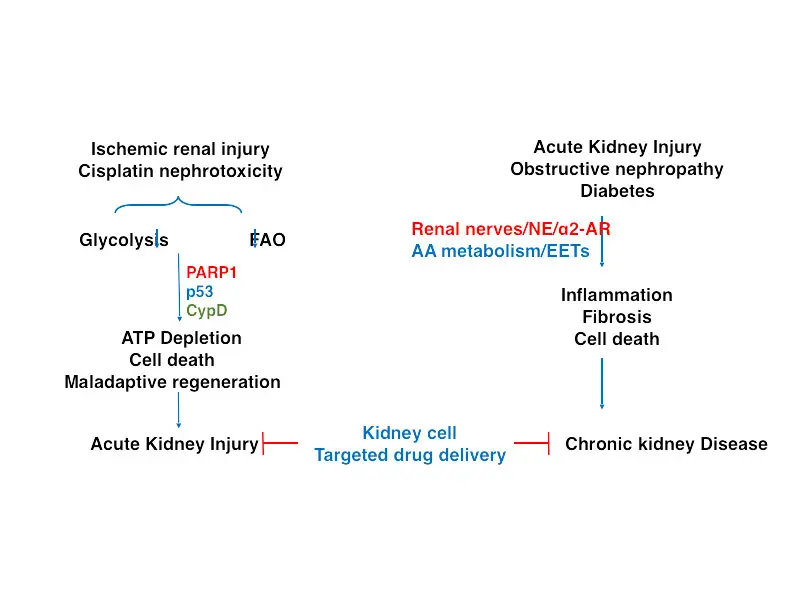
Ischemic renal injury (IRI) and cisplatin nephrotoxicity (CN) are major causes of acute kidney injury (AKI).
For AKI, he is looking at metabolic changes that inhibit glycolysis and fatty acid oxidization—the two main mechanisms by which adenosine triphosphate (ATP) is synthesized in the proximal tubules. He has identified the activation of Poly [ADP-ribose] polymerase 1 (ARP-1) and p53 as factors in inhibiting glycolysis and the activation of p53 and cyclophilin D as factors in inhibiting fatty acid oxidation.
“The hypothesis is that activation of these molecules in the renal proximal tubules after AKI limits their capacity to generate ATP, thus leading to cell death due to energy depletion,” Dr. Padanilam says. “We found that blocking p53 or cyclophilin D in animal models was protective against kidney injury, restoring fatty acid oxidation to normal levels, and thus the proximal tubules’ capacity to produce ATP. The goal is to explore this observation further in clinical trials, and I am working with pharmaceutical scientists to develop therapeutics that can target these specific molecules in the proximal tubules.”
“If we can find the mechanisms that are driving cancer, energy depletion, or nerve regeneration, we can identify therapeutic agents, optimize the conditions, dosage, and route of administration in animal models, and take that into clinical trials,” he says. “If we are successful, we can make a difference in the lives of our patients.”
For CKD, Dr. Padanilam is expanding his initial observation that renal denervation prevents development of renal fibrosis. He is looking at the mechanisms by which the nerve-derived norepinephrine signaling via the alpha 2 adrenergic receptor (α2-AR) induces fibrosis and assessing whether inhibition of α2-AR subtypes or its signaling pathways has therapeutic value in preventing renal fibrogenesis.
“If we can find the mechanisms that are driving cancer, energy depletion, or nerve regeneration, we can identify therapeutic agents, optimize the conditions, dosage, and route of administration in animal models, and take that into clinical trials.”
Babu Padanilam, PhD
“We identified nerve-derived norepinephrine as the driving factor of renal fibrogenesis, signaling via its α2-AR subtype receptors in animal models of CKD,” Dr. Padanilam says. “There are Food and Drug Administration-approved therapeutics for α2-AR, and the plan is to repurpose these drugs and conduct clinical trials to assess the effectiveness of blocking this receptor and thus preventing renal fibrogenesis.”
Ultimately, Dr. Padanilam hopes his efforts to identify the metabolic factors involved in cellular injury and nerve regeneration will have wide-ranging impacts for patients who have kidney, liver, and/or prostate disease.
Featured

Babu Padanilam, PhD
Professor and Director of Kidney Research