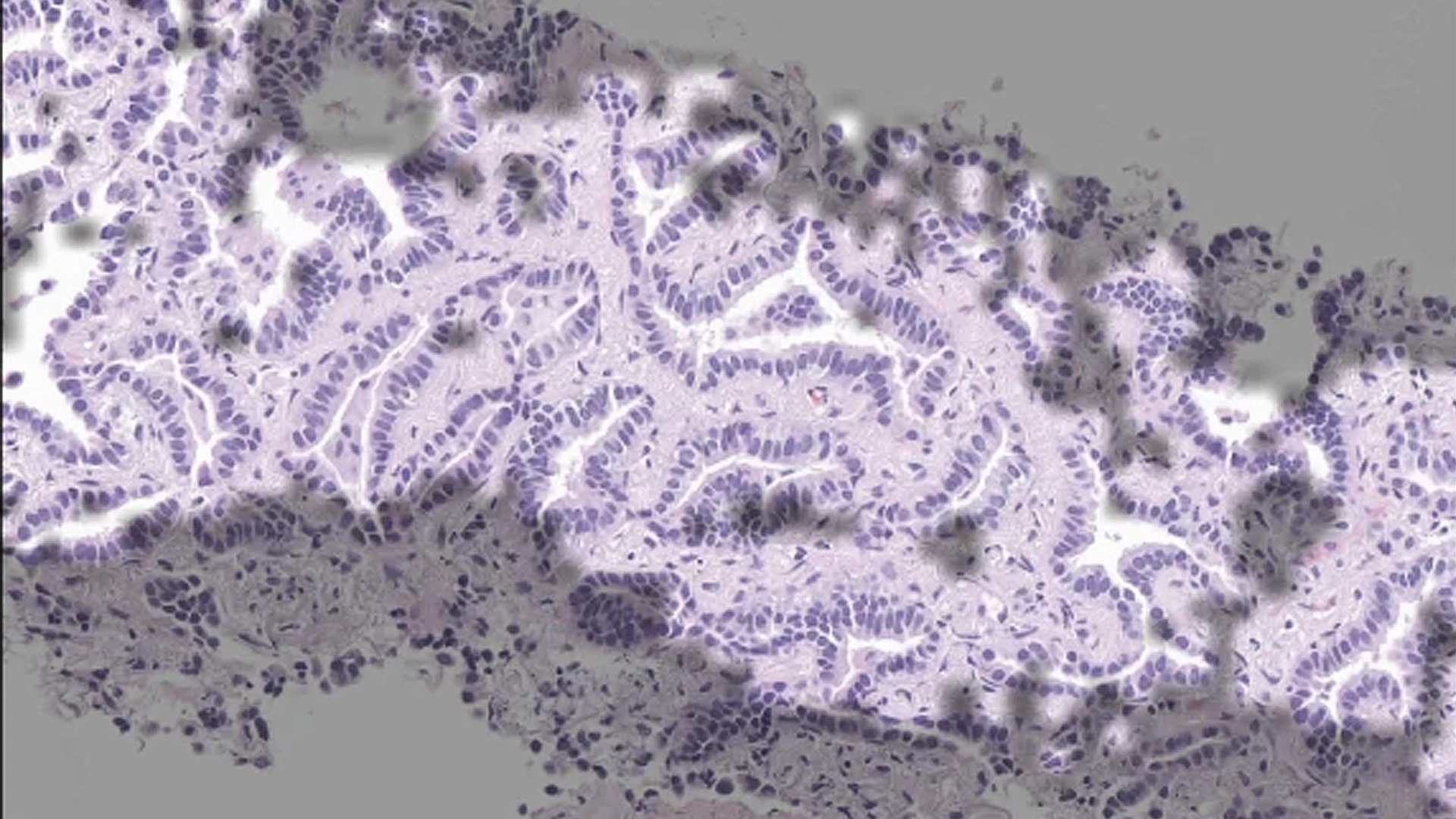
A study by researchers at the Icahn School of Medicine at Mount Sinai, Memorial Sloan Kettering Cancer Center (MSK), and other prominent institutions finds that artificial intelligence could improve lung adenocarcinoma diagnosis, accelerate access to targeted therapies, and reduce reliance on rapid genetic tests.
“Our work shows that AI models can accurately predict some genetic features from routine pathology slides,” says lead author Gabriele Campanella, PhD, Assistant Professor of Artificial Intelligence and Human Health in the Windreich Department of Artificial Intelligence and Human Health at the Icahn School of Medicine.

Gabriele Campanella, PhD, Assistant Professor of Artificial Intelligence and Human Health in the Windreich Department of Artificial Intelligence and Human Health at the Icahn School of Medicine, has developed an AI model that could reliably detect epidermal growth factor receptor (EGFR) mutations in patients with lung adenocarcinoma. The machine learning model aims to overcome standard workflow limitations, where orders for rapid tests compete with next-generation sequencing tests for scarce biopsy tissues.
In a real-time “silent trial,” with findings published in Nature Medicine in July 2025, the researchers showed the approach could reliably detect epidermal growth factor receptor (EGFR) mutations in patients with lung adenocarcinoma. Identifying that biomarker is critical: EGFR mutations can drive tumor growth but also render cancers vulnerable to targeted therapies. The AI model could identify likely EGFR mutations faster than standard rapid genetic tests, without exhausting limited biopsy tissue.
“In some cases, we may be able to replace tissue-exhaustive rapid tests with a computational alternative,” Dr. Campanella says.
The Case for Computational Pathology
Guidelines for lung adenocarcinoma treatment recommend next-generation sequencing (NGS), which can identify mutations relevant for targeted therapies. With NGS results taking several weeks, physicians often request rapid genetic tests to gain early insights to guide treatment in the interim. Because rapid tests provide less information than NGS, and can exhaust biopsy tissue, patients may ultimately require a re-biopsy.
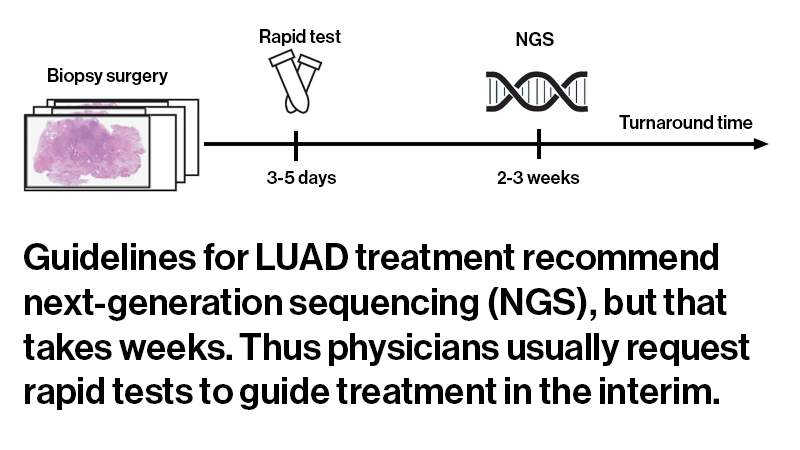
However, rapid tests provide less info than NGS and can exhaust biopsy tissue needed for NGS.
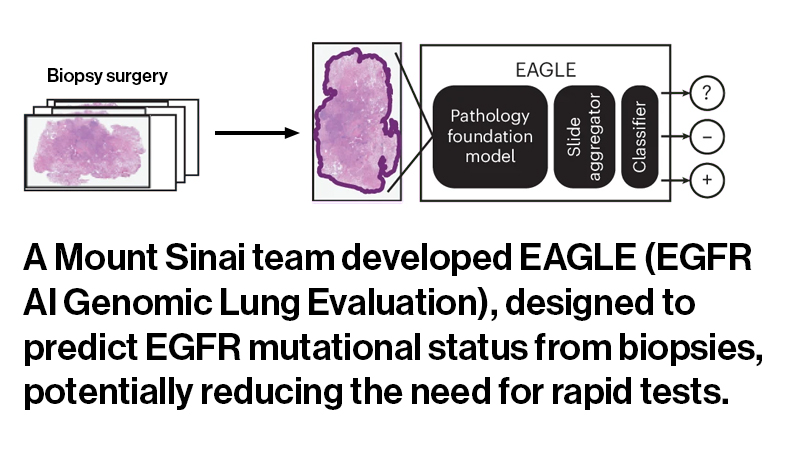
To address these limitations, the team leveraged AI, assembling a clinical dataset of more than 8,000 digital pathology slides from patients with lung adenocarcinoma and fine-tuning an open-source machine learning model to identify patterns associated with EGFR mutations. “Our question was: Can we replace rapid tests, at least under certain conditions, with a computational test to speed results and preserve tissue?” Dr. Campanella says.
To answer that question, he and his colleagues conducted a silent trial at MSK using the AI model to analyze patient samples in real time. Although the predictions were not visible to clinicians and did not influence care decisions, the study demonstrated that AI could reliably detect EGFR mutations.
The researchers evaluated the model under several different scenarios, some more conservative than others. If the AI indicated a high or low likelihood of an EGFR mutation, clinicians could skip the rapid test and proceed directly to NGS. If the probability of a mutation was in the middle, clinicians could proceed with a rapid test while awaiting NGS results. Even in the most conservative model, the computational approach could potentially replace rapid tests in more than 40 percent of cases, without reducing accuracy.
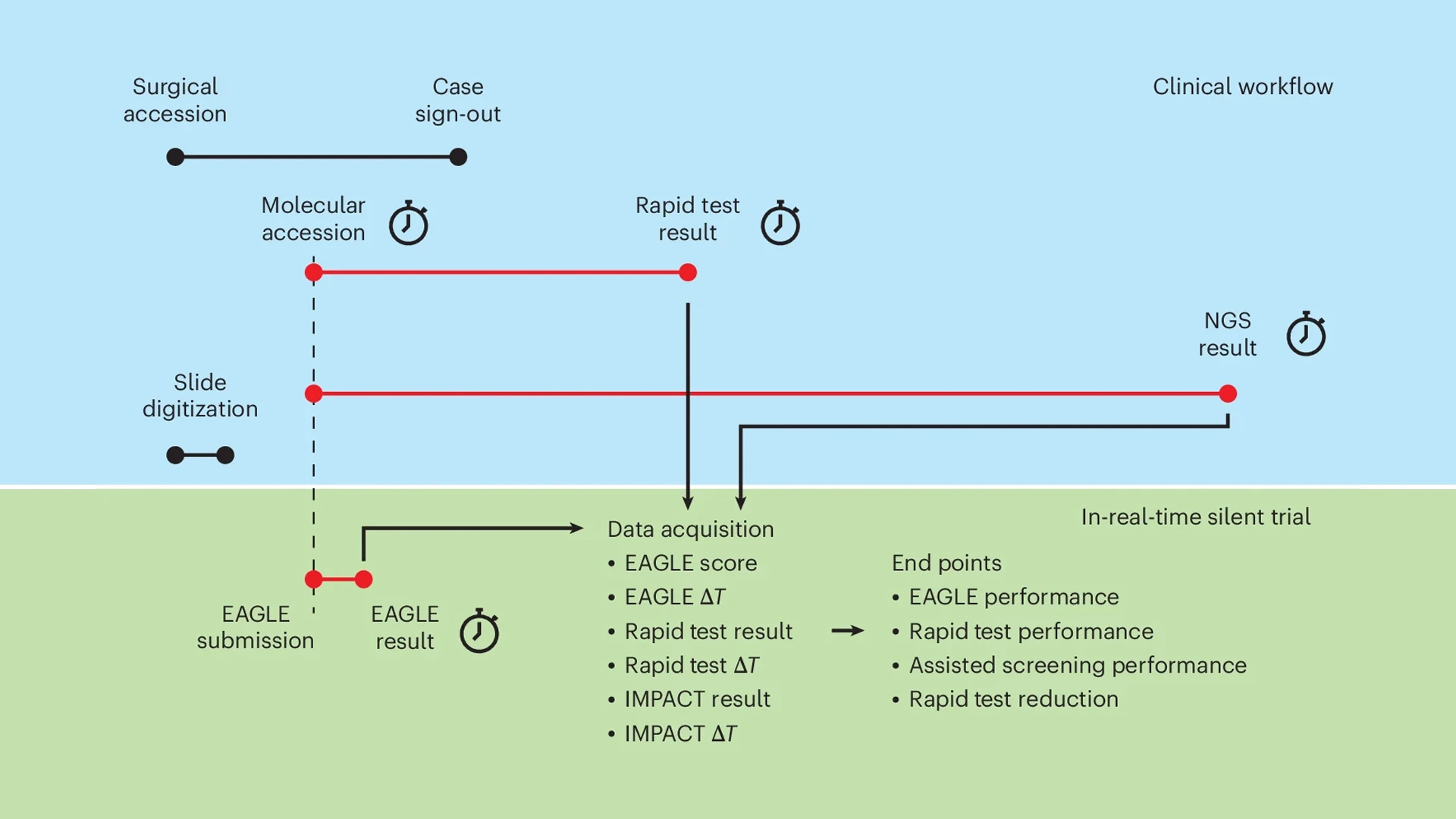
A schematic of the "silent trial" of the EAGLE model. In blue, relevant components of the standard clinical workflow are shown along a timeline. ΔT indicates the time from molecular accession to the availability of a result. The silent trial components occurring in parallel with the clinical workflow are indicated in green.
Notably, the computational approach delivered results in under an hour, compared to about two days for the rapid test. Analyzing additional retrospective data from the United States and Europe, the team showed that the model could be generalized to other populations.
Next Steps: Scaling AI for Clinical Integration
The research team initially focused on EGFR mutations because they comprise a relatively high-volume category of somatic mutations, accounting for about 10 percent of lung adenocarcinomas in the United States, Dr. Campanella says. Following the initial silent trial at MSK, he and his colleagues are preparing to collect additional data at the Mount Sinai Health System. They hope analysis of that data will lay the groundwork for regulatory approval and allow the AI model to be incorporated into the pathology workflow, streamlining biomarker testing and easing pressure on the pathology workforce.
While EGFR mutations are likely just the beginning, the research team is already extending the model to complete lung panels and detect other cancer biomarkers. AI is poised to rapidly accelerate the fields of pathology and oncology, Dr. Campanella and his colleagues predict in a 2025 article in Annals of Oncology.
Mount Sinai, he adds, has emerged as a leader in this effort, having created the first fully digital pathology department in the United States—a shift that has enabled seamless integration of leading-edge tools such as machine learning models. The Health System was also the first in the United States to establish a department dedicated solely to developing patient-centered AI tools, education, and resources—an investment that is further accelerated by close collaboration between clinicians, pathologists, and AI researchers. “By working together, we are developing approaches that will add real value in clinical environments,” Dr. Campanella says.
“While people have tried to develop computational biomarkers for cancer, no one had really pushed it to a clinical scenario,” he adds. “This is the first silent trial done in computational pathology, and we hope it will become the new standard for demonstrating how models such as this can be deployed to improve cancer diagnosis.”
Featured

Gabriele Campanella, PhD
Assistant Professor of Artificial Intelligence and Human Health