While artificial intelligence (AI) has been revolutionizing health care, Claudia Henschke, PhD, MD, Professor of Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai, has also been deploying it to transform her decades-long lung cancer screening program. She and her team outlined in a symposium review article, published in the Journal of Thoracic Imaging in January 2026, the historical milestones that led to their creation of an AI-enabled automated image reading system (AIRS) to detect, measure, and characterize lung nodules.

Claudia Henschke, PhD, MD, Professor of Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai, formed the Early Lung Cancer Action Project (ELCAP), which screened high-risk patients for lung cancer in New York City. That initiative then became the International-ELCAP (I-ELCAP), growing its reach worldwide. Now, empowered by a grant, Dr. Henschke and I-ELCAP are developing an automated image reading system (AIRS), which uses artificial intelligence for nodule detection.
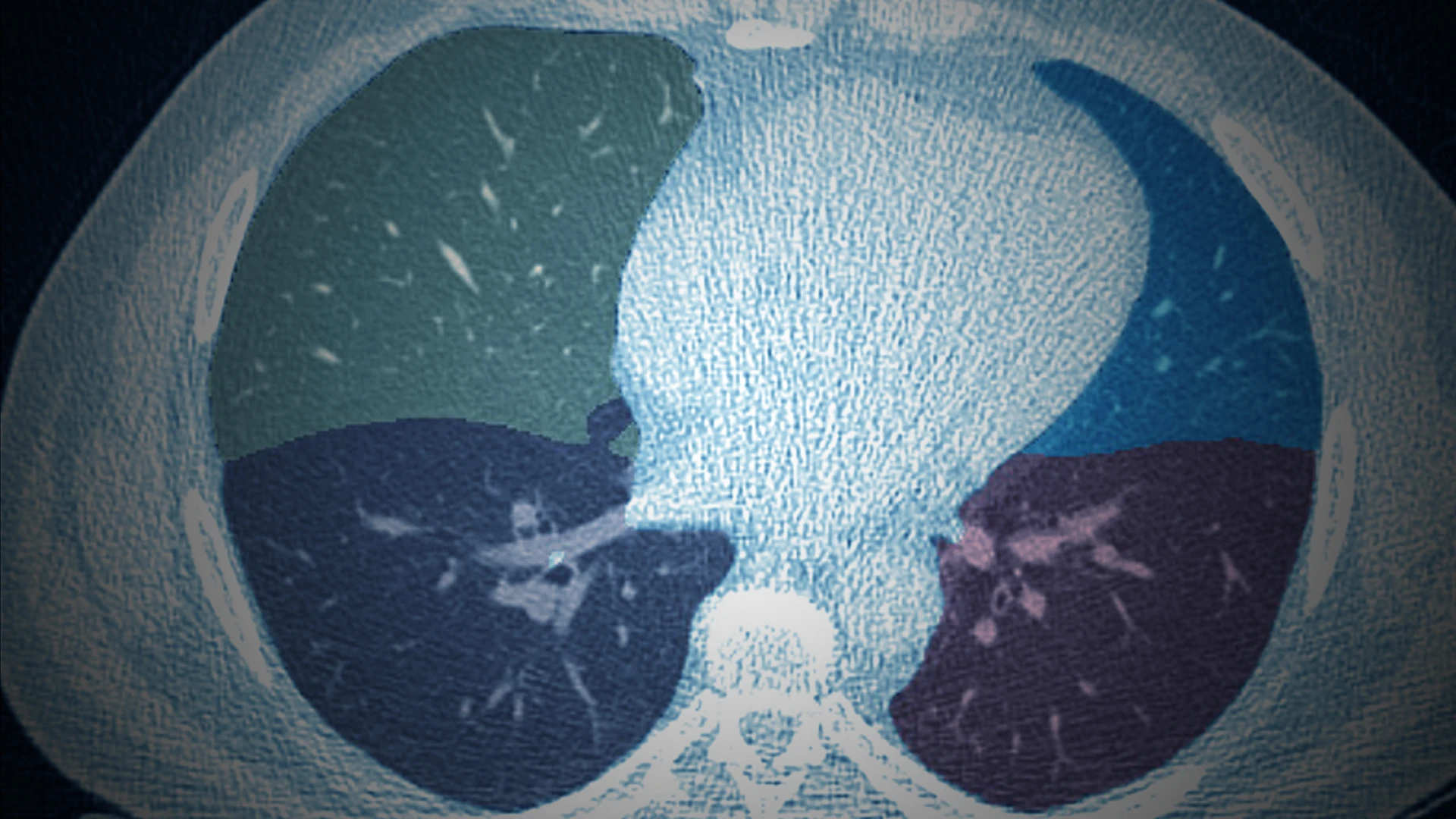
“AIRS is an open-source system for essentially triaging low-dose CT (LDCT) scans. If it finds that an image is unchanged from the prior year—which occurs in the vast majority of scans—it would free up the radiologist to focus on cases where changes have occurred,” says Dr. Henschke. “The improvements in workflow would be significant. And by reducing costs, this so-called AI rule-out system would allow for screening and detection of many more individuals with lung cancer globally.”
Setting the Stage for AI
When helical CT scanners allowed imaging of the entire chest in a single breath-hold in the early 1990s, Dr. Henschke was emboldened by the notion of performing low-dose CT screening for lung cancer. She formed the Early Lung Cancer Action Project (ELCAP) that screened 1,000 high-risk patients at two New York City institutions. From that modest beginning emerged the International-ELCAP (I-ELCAP) and a collaborative team of more than 80 institutions, physicians, and scientists worldwide.
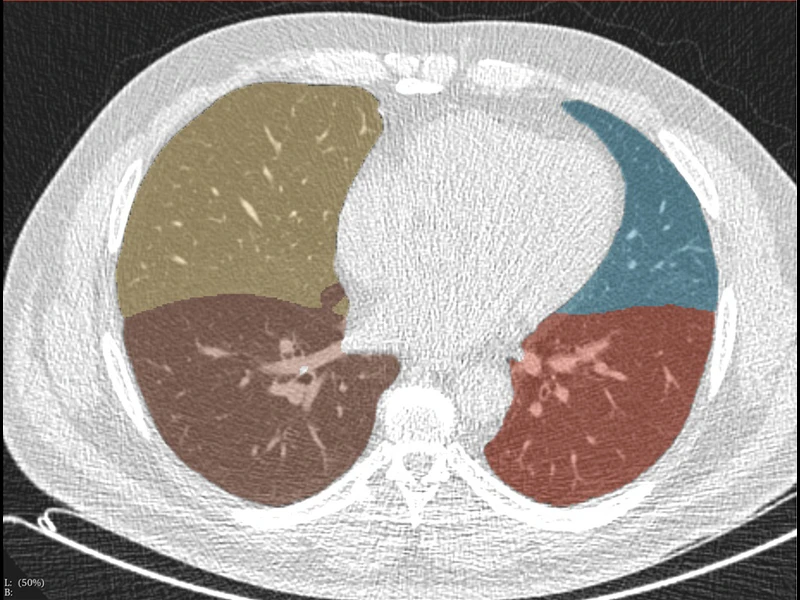
Dr. Henschke and her Mount Sinai team, which includes David Yankelevitz, MD, Professor of Radiology and Director of the Lung Biopsy Service, soon realized that in addition to the early detection of lung cancers, various other pathologies could be identified from the same low-dose CT scan. These include cardiovascular disease and chronic obstructive pulmonary disease (these together cause more premature deaths globally than lung cancer), as well as osteoporosis of the spine and liver disease.
Dr. Henschke and I-ELCAP promote early lung cancer screening, which is something David Yankelevitz, MD, Professor of Radiology, and many physicians at the Mount Sinai Health System have championed. Various departments at Mount Sinai often collaborate to launch screening programs, such as the one shown, to encourage the public to get screened early.
“We knew that performing quantitative analyses of high-resolution CT scans for multiple diseases would require the assistance of AI algorithms,” notes Dr. Yankelevitz, who has worked alongside Dr. Henschke for the past 40 years. “Without that capability, many clinical sites wouldn’t have the radiologic workforce to support lung cancer screening, let alone perform the detection and measurement needed for cardiac and pulmonary disease management, each with different requirements.”
Groundbreaking Role for AIRS
In 2023, the Simons Foundation International awarded Dr. Henschke a $12 million, five-year grant toward the creation of AIRS, which is being evaluated for use as a primary reader for annual repeat and follow-up CT scans. Being a primary reader is groundbreaking as current AI algorithms operate as secondary readers, meaning they provide nodule detection guidance after a radiologist has performed an initial read or, more recently, to operate concurrently with radiologists as they read lung CT scans. AIRS being able to accurately perform as a primary reader would mean freeing a radiologist for other tasks.
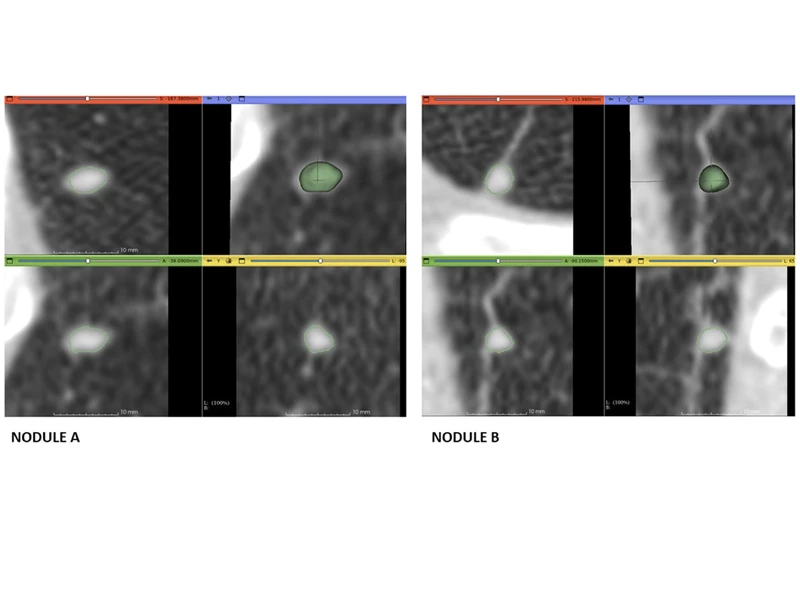
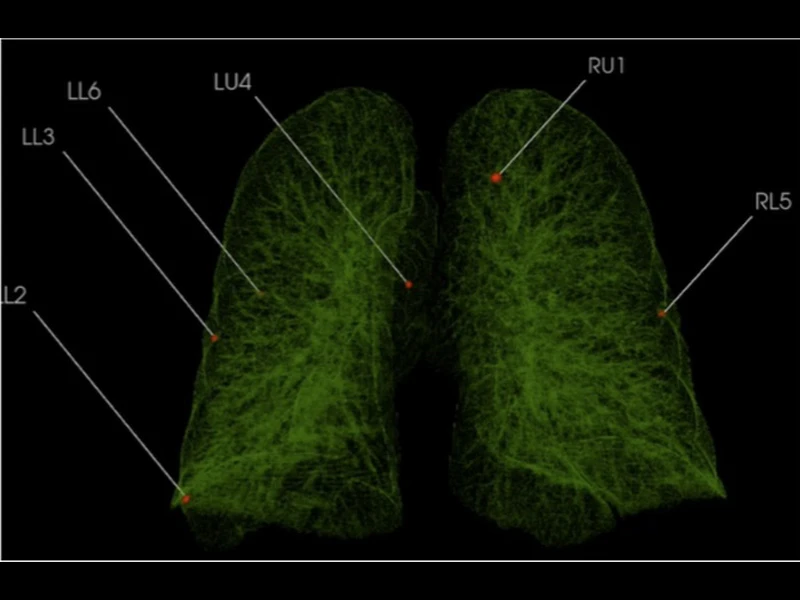
Early results are encouraging. “We have high detection rates of tiny lung nodules as small as four and five millimeters, as well as much larger nodules using data from numerous open-source CT lung imaging databases,” says Dr. Henschke. The goal, she adds, is to improve algorithmic performance rates of AIRS to the point where at least 40 to 50 percent of CT scans can be “ruled out” for further live reviews—the point where radiologists start to see real value, as she puts it.
Ultimately, any primary nodule reading system must be approved by the U.S. Food and Drug Administration, which has granted approval to several secondary and concurrent readers that are now on the market. The agency is just starting to explore primary readings, an area of some controversy since it reduces the need for a radiologist. Dr. Henschke’s team is now working to determine the optimal parameters for these systems, which require exceptionally high sensitivity for nodule detection and extremely low false negatives to be able to rule out further review by a radiologist.
From a Single Scan, a Wealth of Possibilities
AIRS can also be applied to LDCT scans for disease assessments beyond lung cancer, as described in the Journal of Thoracic Imaging paper.
Since the heart is fully visualized on these screens, cardiovascular health is rife with opportunity. Indeed, AI software can automate the detection and scoring of coronary artery calcification from CT scans, potentially enabling earlier and more accurate identification of individuals at risk for heart disease.
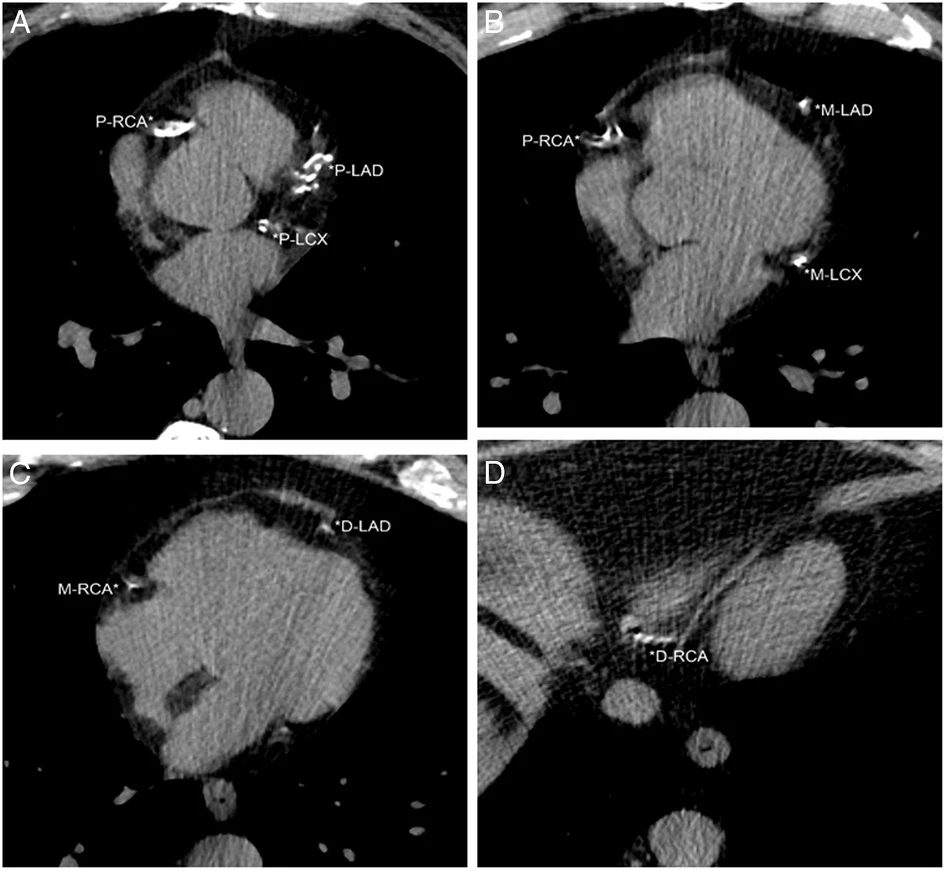
The heart is fully visualized on LDCT screenings for lung cancer, making it suitable for cardiovascular assessments. Dr. Henschke's team developed a visual assessment of coronary artery calcifications (CACs) for LDCT scans that provided a 12-point score by assessing each of the four coronary arteries. In this figure, the patient had an ordinal CAC score of 8 based on calcifications in the proximal left anterior descending (P-LAD), proximal left circumflex (P-LCX), and proximal right coronary arteries (P-RCA) on A, mid left anterior descending (M-LAD) and mid left circumflex (M-LCX) coronary arteries on B, distal left anterior descending (D-LAD) and mid right coronary arteries (M-RCA) on C, and distal right coronary artery (*D-RCA) on D.
Other AI-enabled applications from a single low-dose chest scan include muscle and fat distributions, alcoholic liver disease, nonalcoholic liver disease, hepatic cancers, metabolic disorders, and pancreatic and kidney cancers. Pancreatic cancer is more deadly than lung cancer, but early detection has been shown to greatly increase survival.
“We believe the chest CT will lead to a revolution in the field of preventive health,” says Dr. Yankelevitz, who is a world-recognized expert in fine-needle aspirations of lung nodules. “And a whole new world is developing around it, including advances in digital imaging, new clinical standards, and technologies that will allow patients to simply walk into an upright machine to get scanned. The AI tools we’re developing are very good, and once we have enough high-quality data sets to train them on, there will be virtually no limitations on how far we can take the science.”
Dr. Henschke echoes that thought. “As AI continues to improve, its potential as a powerful, scalable tool for improving population health becomes increasingly clear,” she says. “That’s why ongoing collaboration, standardized protocols, and large annotated datasets—the type of work we’re doing with AIRS—are so critical to advancing the future of integrated, AI-driven preventive care.”
Featured

Claudia Henschke, PhD, MD
Clinical Professor of Diagnostic, Molecular, and Interventional Radiology

David Yankelevitz, MD
Professor of Radiology; Director of Lung Biopsy Service