Patients with muscle-invasive bladder cancer typically undergo surgery to remove the bladder. For about half of them, surgery cures the cancer, but the other half develop metastatic recurrence. The approval of the immune checkpoint inhibitor nivolumab as adjuvant treatment was an important step toward reducing the number of patients who have a recurrence.
Given the risk of side effects from immunotherapy, however, treating patients who are already cured can only cause harm, says Matthew Galsky, MD, Professor of Medicine (Hematology and Medical Oncology), Director of Genitourinary Medical Oncology, and Co-Director of the Center of Excellence for Bladder Cancer at the Mount Sinai Tisch Cancer Center. Yet there has not been a reliable way to tell which patients have microscopic residual cancer and might benefit from the treatment.

Matthew Galsky, MD, Professor of Medicine (Hematology and Medical Oncology), worked on the CheckMate 274 trial, which evaluated whether the immune checkpoint inhibitor nivolumab could prevent cancer from returning in patients with muscle-invasive bladder cancer who had their bladders surgically removed, and is taking that research one step further. The key question now, he says, is knowing which patients actually need the treatment.
Dr. Galsky and fellow researchers at the Icahn School of Medicine at Mount Sinai have tested a new approach to address that conundrum, in a study published in the Annals of Oncology. “Our goal with this study was to identify a test that can tell us who still has cancer in their body and needs additional treatment, and who does not,” says Dr. Galsky.
Initial Validation of the Method
The analysis from Dr. Galsky’s team is linked to the CheckMate 274 trial, which evaluated whether the immune checkpoint inhibitor nivolumab could prevent cancer from returning in patients with muscle-invasive bladder cancer who had their bladders surgically removed. The study showed the drug reduced the risk of recurrence by 30 percent compared with a placebo; this led to the FDA approval of nivolumab as an adjuvant treatment after surgery.
“Nivolumab was the first and so far only immunotherapy treatment approved for bladder cancer in the adjuvant setting,” says Dr. Galsky, who was co-principal investigator and senior author of the CheckMate 274 study, described in the New England Journal of Medicine. “Moving forward, what we really need to know is which patients actually need that treatment.”
The key to that question may be tumor-informed circulating tumor DNA (ctDNA). After tumors were surgically removed, the team used DNA sequencing to identify the unique mutations present in each patient’s cancer cells. “From that genetic fingerprint, a bespoke blood test can be designed to screen for any cancer cells left in a patient’s body that are shedding tumor-specific ctDNA into the blood,” Dr. Galsky says.
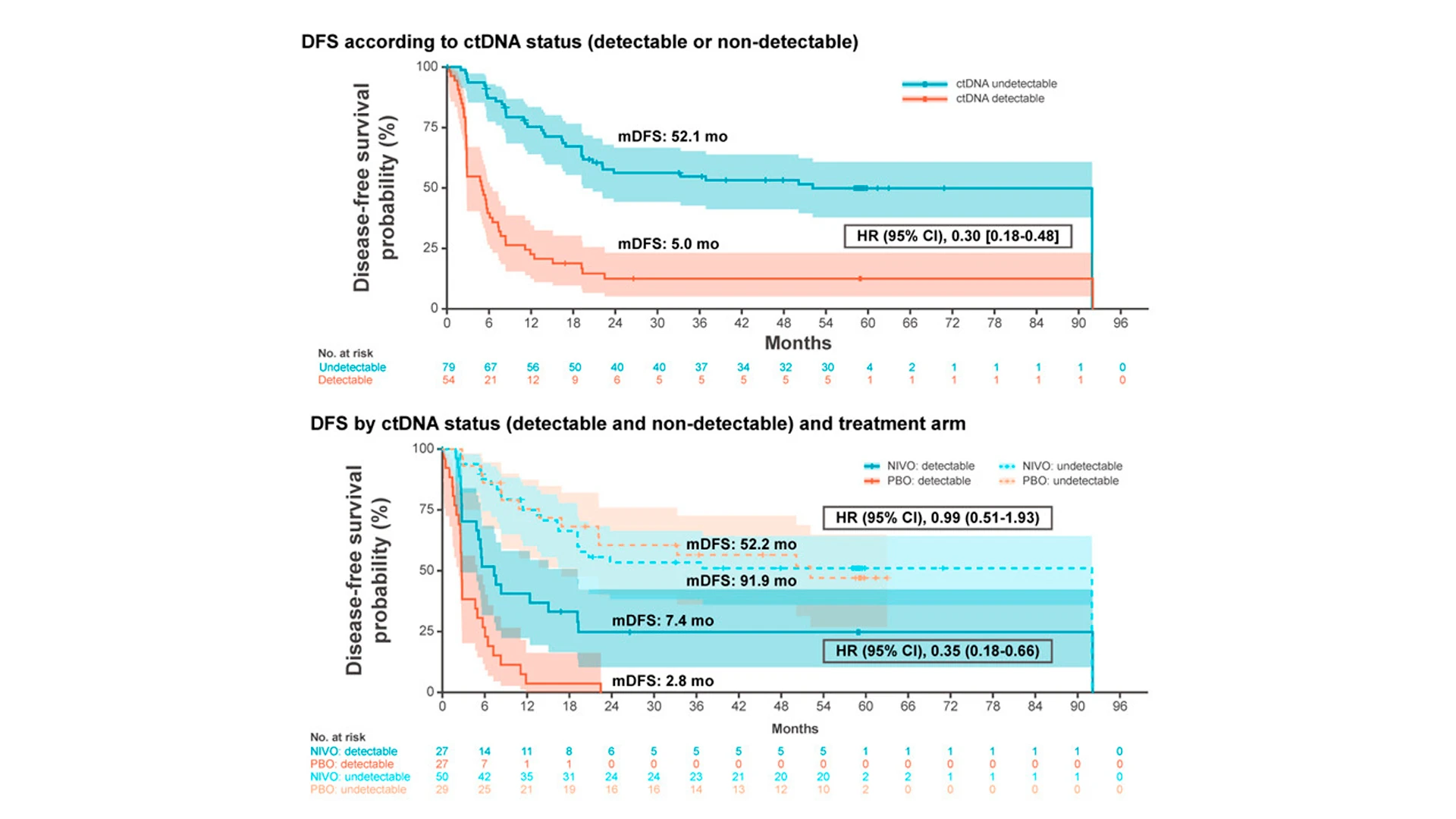
As an exploratory endpoint, Dr. Galsky assessed whether circulating tumor DNA (ctDNA) had any impact on disease-free survival. The team found that patients with nondetectable levels of ctDNA had a 70 percent lower risk of the cancer coming back, compared to those with detectable ctDNA levels. These findings pave the way for leveraging ctDNA as a biomarker for determining which patients might need nivolumab as part of their treatment.
This approach was validated using banked blood specimens from the CheckMate 274 study. At each patient’s first follow-up visit after surgery to remove the bladder, the researchers collected blood samples and tested them for ctDNA. They found that about 40 percent of patients had tumor DNA circulating in their blood—and that marker was a good predictor of cancer recurrence. “There was about a 70 percent lower risk of cancer coming back in patients with undetectable ctDNA compared with those with detectable ctDNA,” Dr. Galsky says.
Because the trial participants were randomized to receive nivolumab or a placebo, the researchers could also assess the relative benefit of immunotherapy in patients with and without detectable ctDNA. Nivolumab reduced the risk of recurrence in about 30 percent of all patients.
“But if we looked just at individuals with detectable ctDNA in the blood after surgery, there was about a 70 percent lower risk of the cancer coming back. When we looked at individuals who did not have ctDNA, it was actually very hard to prove there was any benefit to immunotherapy versus placebo,” Dr. Galsky says. “That is exactly what we would expect to see if the test is doing what we want it to do.”
Using ctDNA to Improve Treatment and Accelerate Research
In next steps, Dr. Galsky is leading a nationwide prospective study with funding from the National Cancer Institute to establish the clinical utility of the ctDNA test for identifying patients at risk of recurrence. The ongoing study focuses on patients with muscle-invasive urothelial cancer who have undergone bladder removal. Those with detectable ctDNA are randomized to receive nivolumab, now the standard of care, or to receive a combination of two immunotherapy drugs. “We chose that approach because even though nivolumab is better than placebo, there is still room for improvement,” Dr. Galsky says.
Patients with undetectable ctDNA are randomized to receive nivolumab or surveillance with the option to start immunotherapy if follow-up ctDNA tests convert from undetectable to detectable. “We’re comparing two approaches: treating everyone regardless of ctDNA status versus treating only those who go on to develop a positive ctDNA test,” he says. “If we can establish the proof of principle for not treating individuals with a negative test, we can spare them the inconvenience, cost, potential side effects, and risks of immunotherapy treatments they don’t really need.”
992
Estimated enrollment size of the MODERN study, an integrated phase 2/3 and 3 trial
Sept 2030
Estimated completion date of the NCI-funded trial
“We need to demonstrate this test is actually leading to better clinical decision-making and better outcomes for patients.”
Matthew Galsky, MD
With the advent of a commercially available test for ctDNA, a personalized medicine approach is within reach. “The field has known for decades that cancer cells shed abnormal DNA into the blood, but it took a long time for the technology to catch up to this idea,” Dr. Galsky says. Now that it has, the implications are wide-ranging, he adds. “One important takeaway from this research is that this approach can be pursued in many different cancer types to identify patients who can benefit from specific treatments.”
While ctDNA tests are being used in other kinds of cancer, most studies so far have focused on clinical validity, Dr. Galsky says. His team hopes their current study, investigating clinical utility, will move the field forward. “We need to demonstrate this test is actually leading to better clinical decision-making and better outcomes for patients,” he says.
The ability to identify ctDNA is also likely to accelerate research. Studies of adjuvant therapies traditionally use disease recurrence as an endpoint. That means researchers must recruit large numbers of patients and monitor them for years until cancer returns—a lengthy and costly research model. Now, researchers can compare two therapies by testing patients for ctDNA at regular intervals to find early signs of recurrence risk.
“Having a new endpoint will expedite the development of new adjuvant treatments,” Dr. Galsky says. “That will enable us to continue to improve treatment options for patients who can benefit the most, without over-treating patients who are effectively cured with surgery.”
Featured

Matthew Galsky, MD
Professor of Medicine (Hematology and Medical Oncology), and Urology