Cancer vaccines hold great promise for treating tumors, but realizing that potential has been a challenge. “Thanks to breakthroughs in whole exome sequencing, which have enabled us to look at mutations in tumor cells at the genomic level, we’re getting closer to that goal,” says Nina Bhardwaj, MD, PhD, Ward-Coleman Chair in Cancer Research. Dr. Bhardwaj is also Director of the Vaccine and Cell Therapy Laboratory and Co-Director of the Cancer Immunology Program at the Mount Sinai Tisch Cancer Center.

Nina Bhardwaj, MD, PhD, Ward-Coleman Chair in Cancer Research at the Icahn School of Medicine at Mount Sinai, is exploring the use of a novel multi-peptide neoantigen cancer vaccine, PGV001, in studies of several cancer types. The findings represent a big step forward in personalized cancer treatment, holding potential to activate a patient’s immune response to boost the efficacy of existing immunotherapies.
Dr. Bhardwaj and her colleagues at the Mount Sinai Tisch Cancer Center are advancing the field despite those challenges, progressing toward the goal of creating a truly personalized cancer vaccine. In a pair of phase 1 trials, the team demonstrated that their novel multi-peptide neoantigen cancer vaccine, PGV001 (personalized genomic vaccine 001), was feasible and safe and triggered an immune response in patients with a variety of solid tumor and hematologic cancers.
Laying the Groundwork With a Computational Pipeline
As tumor cells mutate, they accumulate changes to their genomes that lead to the production of novel proteins. “The immune system has not really seen those proteins before and may potentially recognize them as antigens,” says Dr. Bhardwaj, who is also Professor of Medicine (Hematology and Medical Oncology) and Urology. Using OpenVax, a computational platform designed by experts at the Icahn School of Medicine at Mount Sinai, the scientists can analyze tumor and germline data to select the most promising novel antigens, or neoantigens, to target. Then, they can create a personalized vaccine to train the patient’s immune system to recognize and mount a response against those unique targets.
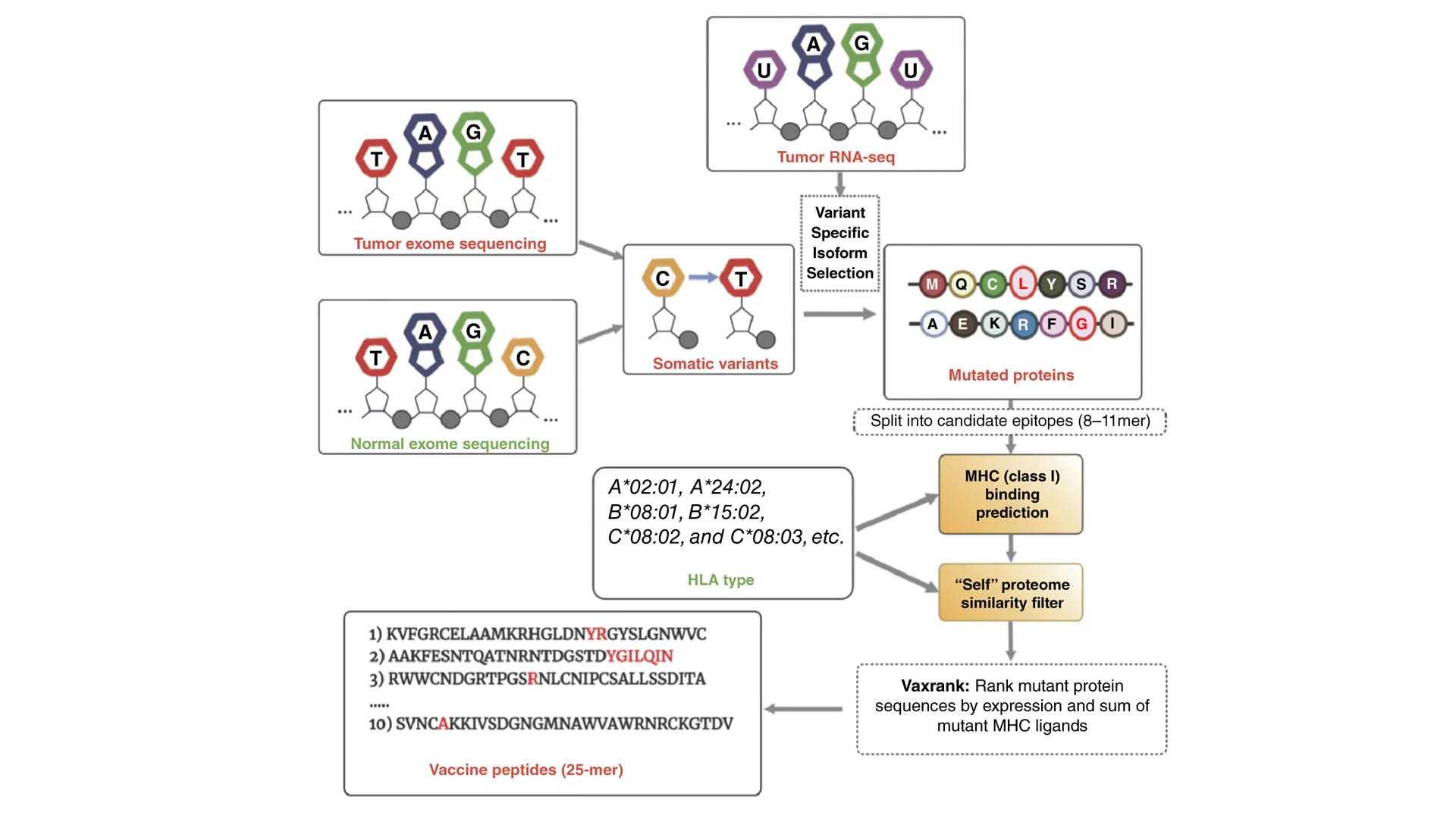
PGV001 neoantigens are predicted using the OpenVax platform, which involves a workflow that begins with identifying somatic variants from tumor/normal exome sequencing, seen above. For each identified mutation with a predicted coding effect—missense, frameshift, or stop loss—tumor RNA sequencing (RNA-seq) is used to confirm expression and correctly phase the somatic mutation with any nearby germline polymorphisms. The platform then generates several candidate vaccine peptides, which are analyzed for binding to a patient's HLA class I alleles.
Using this methodology, a team of experts in immunology, oncology, genetics, and pathology has completed two separate phase 1 trials: one that examined a range of cancer types and another that focused on patients with bladder cancer. The encouraging results are already leading to new research to develop and test personalized cancer vaccines.
Basket Trial for Cancers With High Risk of Recurrence
In the first phase 1 trial of PGV001, the researchers opted for a basket trial designed to explore the safety, tolerability, and immunogenicity of the personalized vaccine across several types of cancers, including multiple myeloma and cancers of the lung, breast, bladder, and head and neck. “The rationale for doing a basket trial was that this method could theoretically work for any cancer type. So, we chose to focus on patients at high risk of recurrence,” Dr. Bhardwaj says.
They recruited 13 patients who had received standard treatments for their diseases. The patients were all in remission with no metastases at the beginning of the study, but at high risk of recurrence based on factors such as their cancer type and the molecular features of the cancer.
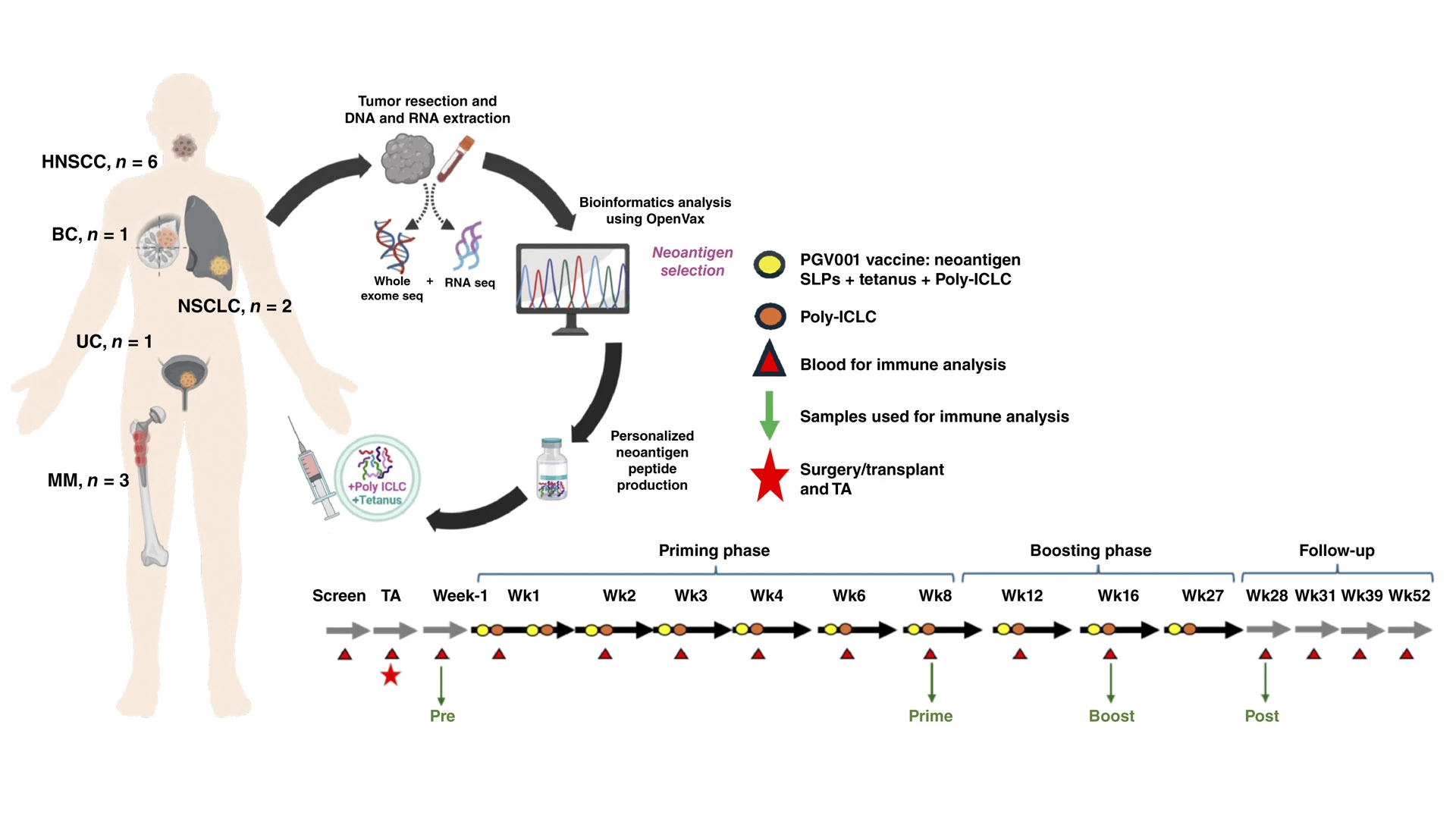
The schematic above demonstrates the phase 1 trial of PGV001, a basket trial with different cancer types and a number of patients with each type: head and neck squamous cell carcinoma (6), multiple myeloma (3), non-small cell lung cancer (2), breast cancer (1), and urothelial cancer (1). Participants had their tumors resected, sequenced, and developed into a personalized cancer vaccine. They were then treated with the vaccine and followed up—up to 52 weeks—for safety, as well as T-cell and B-cell responses.
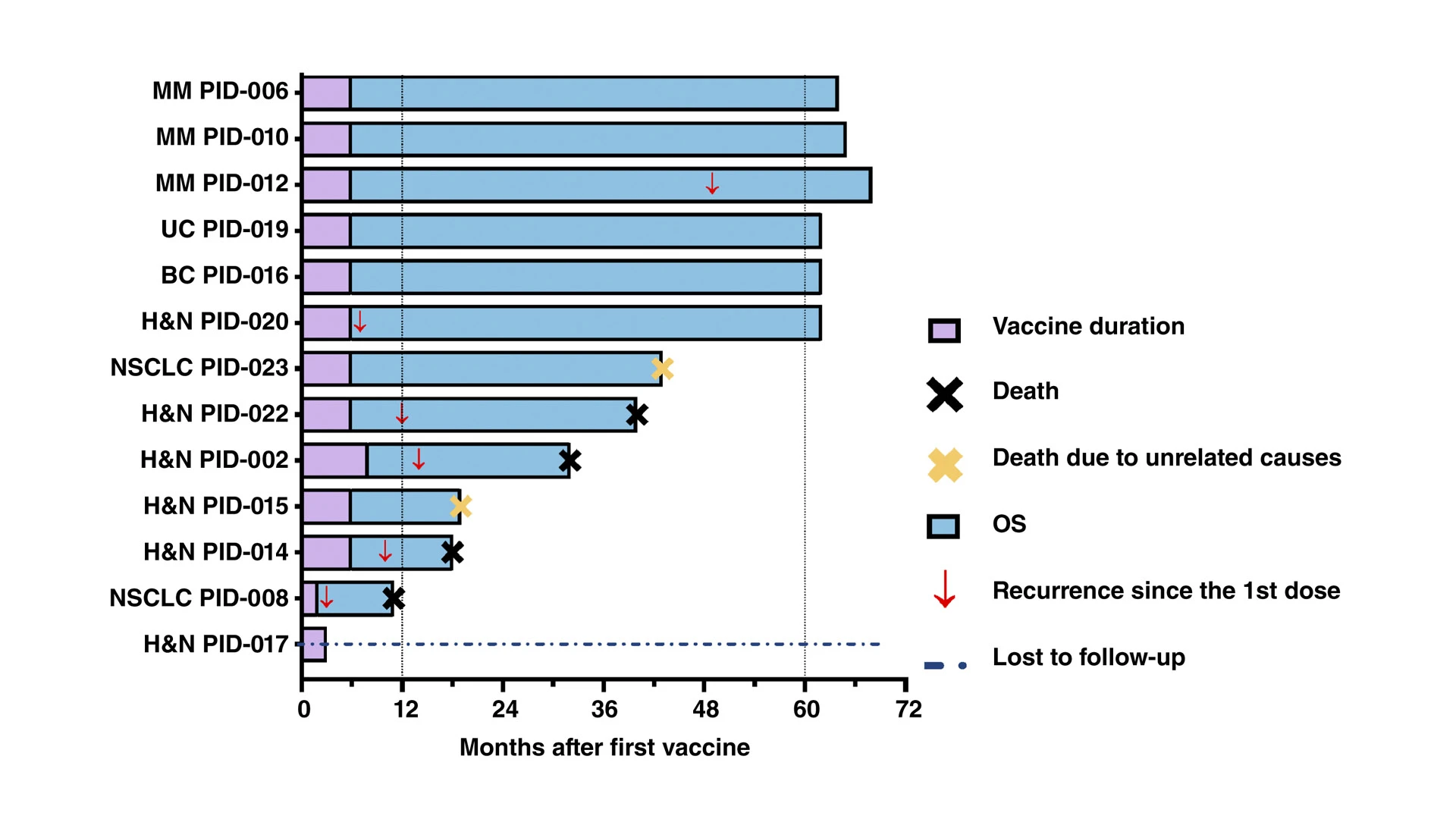
At the 1-year follow-up, 11 of 13 patients were alive, one patient was lost to follow up, three had disease recurrence, and one had died from disease recurrence. At 60 months after the first vaccination, two additional patients had experienced disease recurrence. Overall, six patients were alive and six were deceased at five years follow-up. Notably, two additional patients had expired without documented evidence of disease recurrence; they died of etiologies not related to their cancer. The median overall survival (OS) was 51.5 months, whereas the median recurrence-free survival (RFS) calculated from the time of curative intent treatment was 49 months.
The results, published in May 2025, in Cancer Discovery, showed that PGV001 was feasible and safe, with no serious side effects. The most common adverse events were mild injection site reactions, fatigue, and fever. While safety and feasibility were the primary endpoints of the study, the researchers also looked for evidence of immunogenicity. Dr. Bhardwaj and her colleagues found encouraging evidence that the therapy was having the desired effect.
Of the 11 patients who completed the treatment, all developed targeted T-cell and B-cell responses. In other words, the OpenVax platform successfully predicted unique immunogenic neoantigens for each patient, and the resulting vaccines generated an effective immune response.
At the five-year follow-up, six of the patients survived, and three were cancer-free. While the trial was too small to draw conclusions about improved survival, Dr. Bhardwaj says, there were encouraging signs that patients experienced possible clinical benefits.
Improving Immunotherapies for Bladder Cancer
Immunotherapy treatments have significantly advanced urothelial cancer treatment, but not all patients respond, and others develop resistance to the therapies. One hope is that a personalized vaccine could activate a patient’s immune response to boost the efficacy of existing immunotherapies.
In a second phase 1 trial, Dr. Bhardwaj and colleagues, including Matthew Galsky, MD, Co-Director of the Center of Excellence for Bladder Cancer and Deputy Director of the Mount Sinai Tisch Cancer Center, studied PGV001 in patients with bladder cancer. Following standard treatment, patients received the personalized vaccine combined with the immune checkpoint inhibitor atezolizumab.
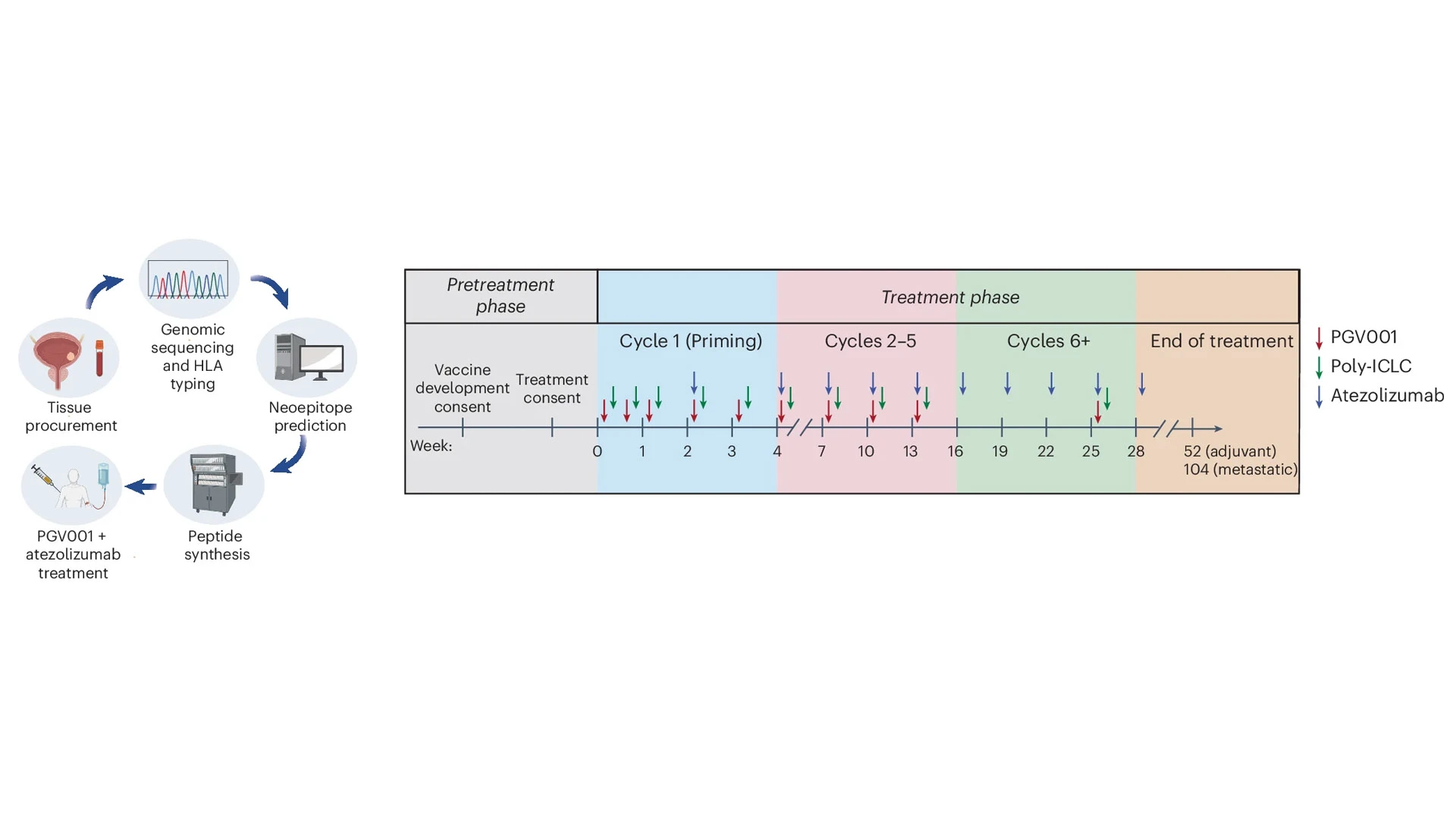
In this phase 1 trial, patients with urothelial cancer received PGV001 treatment combined with the immune checkpoint inhibitor atezolizumab. Of the participants, 10 initiated the treatment phase, with four in the adjuvant setting and six in the metastatic setting.
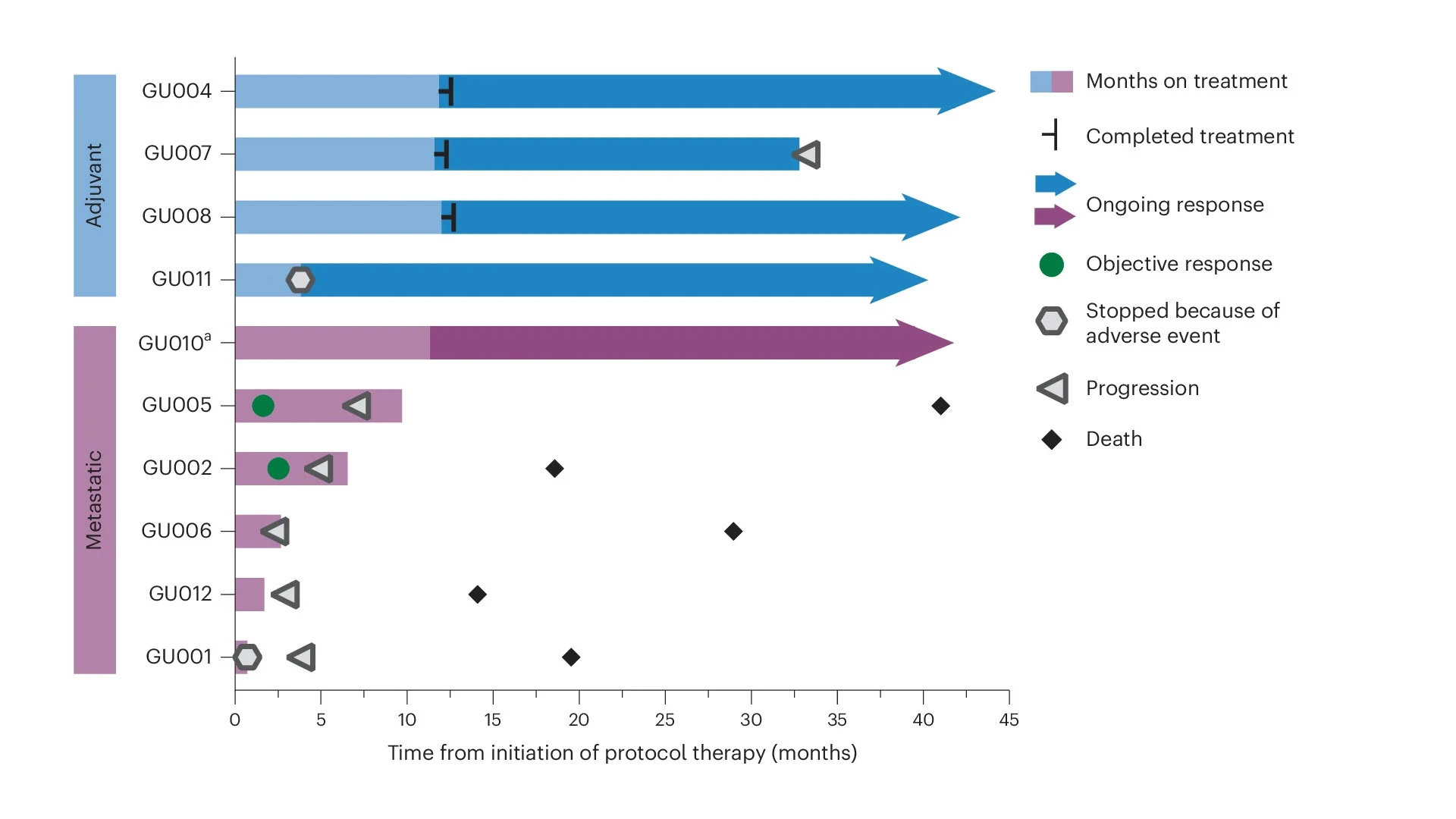
In the adjuvant setting, three of the four participants did not have progression of disease. In the metastatic setting, two participants had an objective response in the metastatic sites. These early findings are promising as they suggest different settings in which cancer vaccines can be combined with immunotherapy, says Dr. Bhardwaj.
The researchers treated 10 patients with PGV001 in addition to atezolizumab. Some originally had muscle-invasive disease and were treated in the adjuvant setting, while others had disease that progressed to metastasis.
Again, side effects were minor, and the treatment generated a strong immune response in all of the participants. At a median follow-up of 39 months, three out of four patients treated in the adjuvant setting were free of recurrence. Among the five participants with metastatic disease, two showed a partial response of disease in the metastatic sites. The results were published May 9, 2025, in Nature Cancer.
New immune-based therapies are being developed every year, changing the way cancers, such as bladder cancer, are treated. In some cases, checkpoint therapies for bladder cancer are now used before or in combination with first-line chemotherapy, pointing toward the possibility of using personalized vaccines earlier in the treatment process. Other novel immunotherapy treatments have been approved for treating metastatic bladder cancer, which might benefit from a personalized immune system boost. “There are multiple settings where we could combine vaccination to improve the immune response, and that’s an exciting direction for future research,” Dr. Bhardwaj says.
Next Steps for PGV001
Studies are underway to test PGV001 in patients with aggressive prostate cancer and glioblastoma. That may be just the beginning. In bladder cancer, Drs. Bhardwaj and Galsky hope to test personalized vaccines combined with fibroblast growth factor receptor (FGFR) inhibitors, which inhibit pathways that promote tumor growth and metastasis.

Dr. Bhardwaj (left), Kazuki Sone, PhD (right), and a team of researchers at the Icahn School of Medicine, are currently running trials of PGV001 in prostate cancer and glioblastoma. There are opportunities for combining the cancer vaccine with other interventions, including with fibroblast growth factor receptor (FGFR) inhibitors for bladder cancer, or with cytokine immune modulators for hematologic cancers.
In blood cancers, Dr. Bhardwaj envisions giving the vaccine alongside antibodies that target mutated receptors. The treatment could also be given alongside immune modulators such as cytokines or chemokines, she says, to help immune cells get to the places they need to go to generate a powerful immune response.
“It’s exciting because we now have a much bigger armamentarium. We have new targets and new vaccine platforms, such as RNA vaccines,” she says. “We’re thinking about future iterations of where to put vaccination, and we have a lot of options.”
In the process, she and her colleagues are learning more about many different classes of neoantigens, many of which have not been well studied—and that could eventually serve as potential treatment targets.
Researchers are also starting to better understand the tumor microenvironment in bladder cancer and other cancer types. “Often, the tumor microenvironment promotes cancer cell growth while limiting the function of immune cells,” Dr. Bhardwaj says. As researchers learn more, they may be able to develop therapies that make the tumor microenvironment more conducive to treatments such as personalized cancer vaccines.
Quicker Access Thanks to Multidisciplinary Effort
One challenge of developing personalized vaccines is the significant time it takes to produce the tailored treatments. In the bladder cancer trial, it took a median time of 20 weeks to prepare each unique vaccine. That timeline has shrunk considerably as technology advances, and vaccine platforms using peptides can be created more efficiently, Dr. Bhardwaj says.
20 weeks
Median time taken to prepare each vaccine in the bladder cancer trial
As technology improves, the timeline to prepare personalized vaccines is likely to come down.
Still, the research team is mindful that personalized medicine takes time and resources. One way to make personalized vaccines more time- and cost-effective may be to target groups rather than individuals, Dr. Bhardwaj says. In a related line of research, she and her colleagues are exploring the development of a neoantigen vaccine that is personalized to a group of people whose cancers share mutations in the same gene. Such a product may combine the benefits of individualized treatments with the ease of off-the-shelf products.
Dr. Bhardwaj, an immunologist by training, says the progress of personalized cancer vaccines has been possible thanks to the culture of collaboration at Mount Sinai that brings together experts in specialties such as immunology, oncology, and genetics. The team comprises multiple researchers from the Icahn School of Medicine, including Thomas Marron, MD, PhD; Matthew Galsky, MD; Mansi Saxena, PhD; Jonathan Anker, MD, PhD; Ashutosh Tewari, MD; Ronald Hoffman, MD; and others.
The research also owes its early successes to the establishment of the Mount Sinai Vaccine and Cell Therapy Laboratory, an innovative facility that enables the team to manufacture vaccines and cellular therapies for phase 1 and 2 studies onsite in a dedicated, controlled environment. “The lab has been foundational to our ability to undertake these studies,” Dr. Bhardwaj says, and she and her colleagues are eager to build on that foundation.
Featured

Nina Bhardwaj, MD, PhD
Ward Coleman Chair in Cancer Research