There are around 35,000 new cases of multiple myeloma a year in the United States, yet research has yielded few significant improvements in long-term clinical benefits or survival. That outlook has brightened in recent years with the development and approval of immunotherapies directed against B cell maturation antigen (BCMA) expressed on plasma cells.
These agents include chimeric antigen receptor T cell (CAR T cell) therapy and bispecific antibodies, which are producing unprecedented response rates in patients with relapsed/refractory multiple myeloma. These interventions are promising to transform the treatment paradigm, and Mount Sinai is a nationally recognized leader in the field, playing a pivotal role in most of the studies investigating new and innovative modes of treatment.
Findings from one of those studies, CARTITUDE-1, reported in June 2025, showed that 33 percent of patients with multiple myeloma remained progression free for five or more years after a single injection of CARVYKTI, a CAR T cell treatment.

Some of the treatment options for multiple myeloma that Mount Sinai researchers have been investigating are truly making a significant difference, with some coming close to being considered a cure, said Sundar Jagannath, MBBS.
“To have one-third of late-stage multiple myeloma patients who would normally be going to hospice actually be alive and free of cancer progression after five years is truly remarkable,” says Sundar Jagannath, MBBS, Professor of Medicine (Hematology and Medical Oncology) at the Icahn School of Medicine at Mount Sinai, and Principal Investigator of CARTITUDE-1.
The Mount Sinai team has also observed never-before-seen responses in myeloma patients with bispecific antibodies, and is playing a pivotal role in uncovering next-generation interventions.
CARVYKTI Changes the Treatment Landscape
CARVYKTI works by harnessing a patient’s immune system to directly attack the cancer and keep it from coming back. T cells are removed from the patient’s blood and genetically modified in the lab to recognize and attack BCMA, a protein that is overexpressed on the surface of nearly all multiple myeloma cells.
The engineered T cells—or CAR T cell therapy—are returned to the body through a one-time infusion, and rapidly multiply. The entire therapy process from collection to post-infusion takes two to three months.
CARVYKTI was approved by the U.S. Food and Drug Administration (FDA) in April 2024 for patients who had received one prior line of standard treatment, including a proteasome inhibitor or an immunomodulatory agent. In the CARTITUDE-1 trial, where Mount Sinai was a major recruiting site, researchers tracked patients treated with the CAR T cell therapy and saw superior overall survival and progression-free survival compared to standard of care. The results were presented at the American Society of Clinical Oncology 2025 Annual Meeting and published in June 2025 in the Journal of Clinical Oncology.
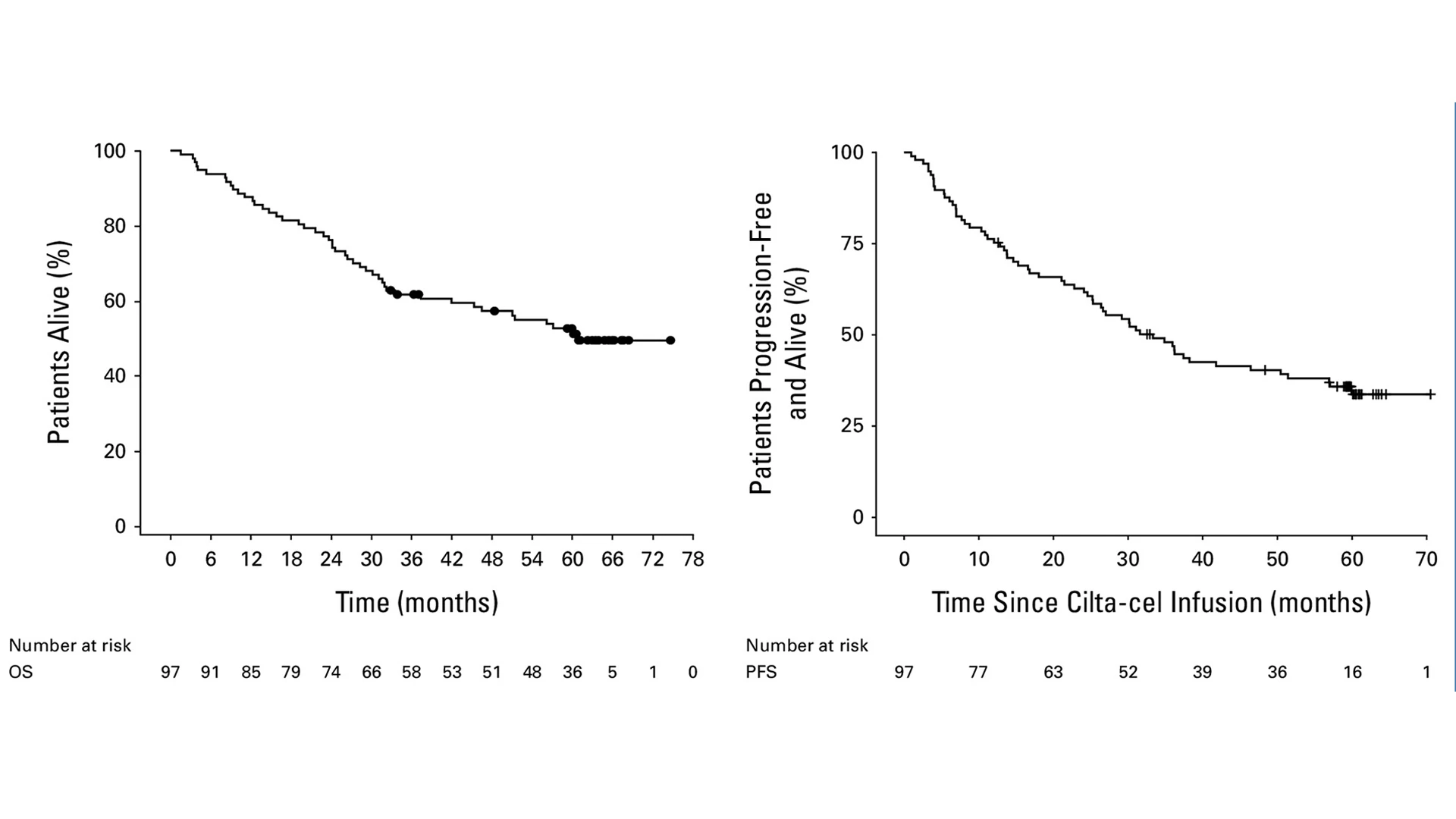
In long-term survival data of the CARTITUDE-1 trial presented at the American Society of Clinical Oncology 2025 Annual Meeting, of 97 patients who received treatment with CARVYKTI, 45 (46.4%) were alive and in long-term follow-up. Median overall survival was 60.7 months, seen in the left graph. The right graph shows progression-free survival.
1 year
Life expectancy of multiple myeloma patients before availability of CARVYKTI
In addition to the groundbreaking results showing that a third of participants remained cancer-free for five years or more with no further multiple myeloma treatment required, CARTITUDE-1 reported that nearly half of all participants were still alive at five years and beyond when their life expectancy prior to the trial was just one year.
“The durability and consistency we saw with CARVYKTI in our study offer real hope for long-term disease control in a population that previously had very limited options,” said Dr. Jagannath.
CARVYKTI is just one of many doors Mount Sinai is opening in collaboration with pharmaceutical and other research partners to better understand and treat multiple myeloma. Mount Sinai researchers are engaged in clinical trials for allogeneic CAR T cell technology, for example, where T cells are removed from young, healthy individuals, prepared for the fight against multiple myeloma through CRISPR gene editing, and made available as an off-the-shelf agent to patients whose own T cells are not up to the challenge. In addition, Mount Sinai researchers are identifying, through genomic sequencing of tumor cells and the study of immune cells, patients most likely to benefit from CAR T cell therapy, as well as those who might be vulnerable to its side effects.
The Promise of Bispecific Antibodies
Some of the most promising research in the fight against multiple myeloma is taking place in the realm of bispecific antibodies (BsAbs). These compounds have two arms that bind to both the cancer-fighting T cell and the protein expressed on the surface of the multiple myeloma cell, which can be either BCMA or G protein-coupled receptor class C group 5 member D (GPRC5D). This dual attack strategy boosts the immune response and offers an advantage over traditional therapies in seeking out and destroying myeloma cells.
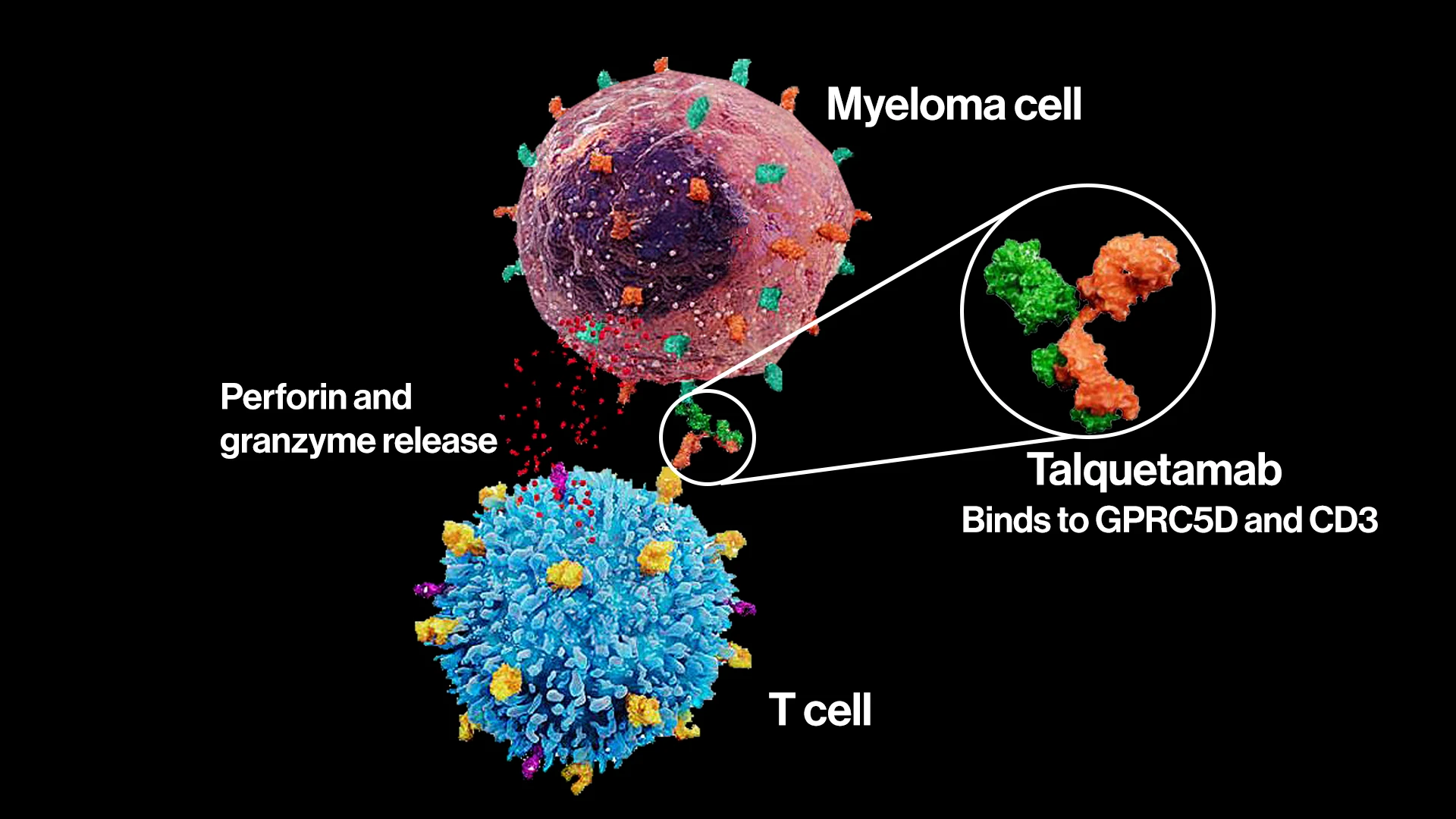
Talquetamab is one of the bispecific antibodies researched at the Icahn School of Medicine. This molecule binds to both GPRC5D—found on the surface of myeloma cells—and CD3—a receptor on T cells. The dual affinity signals a strong release of cytotoxic proteins, including perforin and granzymes, to induce myeloma cell death.
Mount Sinai has been an early player in the field of BsAbs, spearheading clinical trials for talquetamab, which received FDA approval in August 2023 for patients with relapsed/refractory multiple myeloma. Talquetamab, which targets T cell surface antigen CD3 and GPRC5D, has shown strong cytotoxic T lymphocyte response.
From their findings on BsAbs, Mount Sinai researchers are already looking at what lies beyond: trispecific antibodies. Currently in phase 1 trials, this next-generation agent latches on to the cancer cell more effectively by binding to BCMA and GPRC5D, and additionally to T cells, thus improving the reach and effectiveness of the intervention.
“Because of this extraordinary binding power, we’ve seen a response rate of 95 percent in myeloma patients given trispecific antibodies, and with minimal toxicity,” said Dr. Jagannath. “We believe this could be a real game-changer for the field once trispecific antibodies are approved, possibly in another two years.”
Bispecific Antibodies Vs. CAR T-Cell Therapy
In the meantime, as BsAbs and CAR T cell therapies increasingly become mainstays, oncologists will have to determine which intervention is best suited for a patient.
Some myeloma patients who are not eligible or suitable for CAR T cell therapy might be good candidates for treatment with BsAbs, said Dr. Jagannath. BsAbs have an advantage of greater accessibility since, unlike CAR T cell therapy, they don't have to be uniquely engineered to the patient—a process that can take months. BsAbs could be made available quickly as an off-the-shelf medication, meaning there would be no delay in starting treatment.
“CAR T cell therapy is the only treatment I’ve given that actually resulted in no trace of cancer after five years. No other drug to date has shown that kind of curative potential for multiple myeloma—and that’s a very powerful statement.”
- Sundar Jagannath, MBBS
A major downside, though, is that BsAbs must be administered and closely monitored for at least several years, and hospitalization could be required for initial dosing. CAR T cell therapy, on the other hand, is a one-and-done treatment through a single injection.
Is one treatment considered better than the other? Dr. Jagannath is circumspect on that question, citing the advantages of each of these innovative therapies. However, he noted, “CAR T cell therapy is the only treatment I’ve given that actually resulted in no trace of cancer after five years. No other drug to date has shown that kind of curative potential for multiple myeloma—and that’s a very powerful statement.”
Featured

Sundar Jagannath, MBBS
Professor of Medicine (Hematology and Medical Oncology)