The INO80c chromatin remodeling complex is often mutated in several different types of cancers, but little is known about its role in cancer pathogenesis or immune cell dysfunction. David Dominguez-Sola, MD, PhD, Associate Professor of Oncological Sciences, and Pathology, and his team are working on understanding these mutations that could ultimately lead to novel treatments for certain rare but fatal cancers.
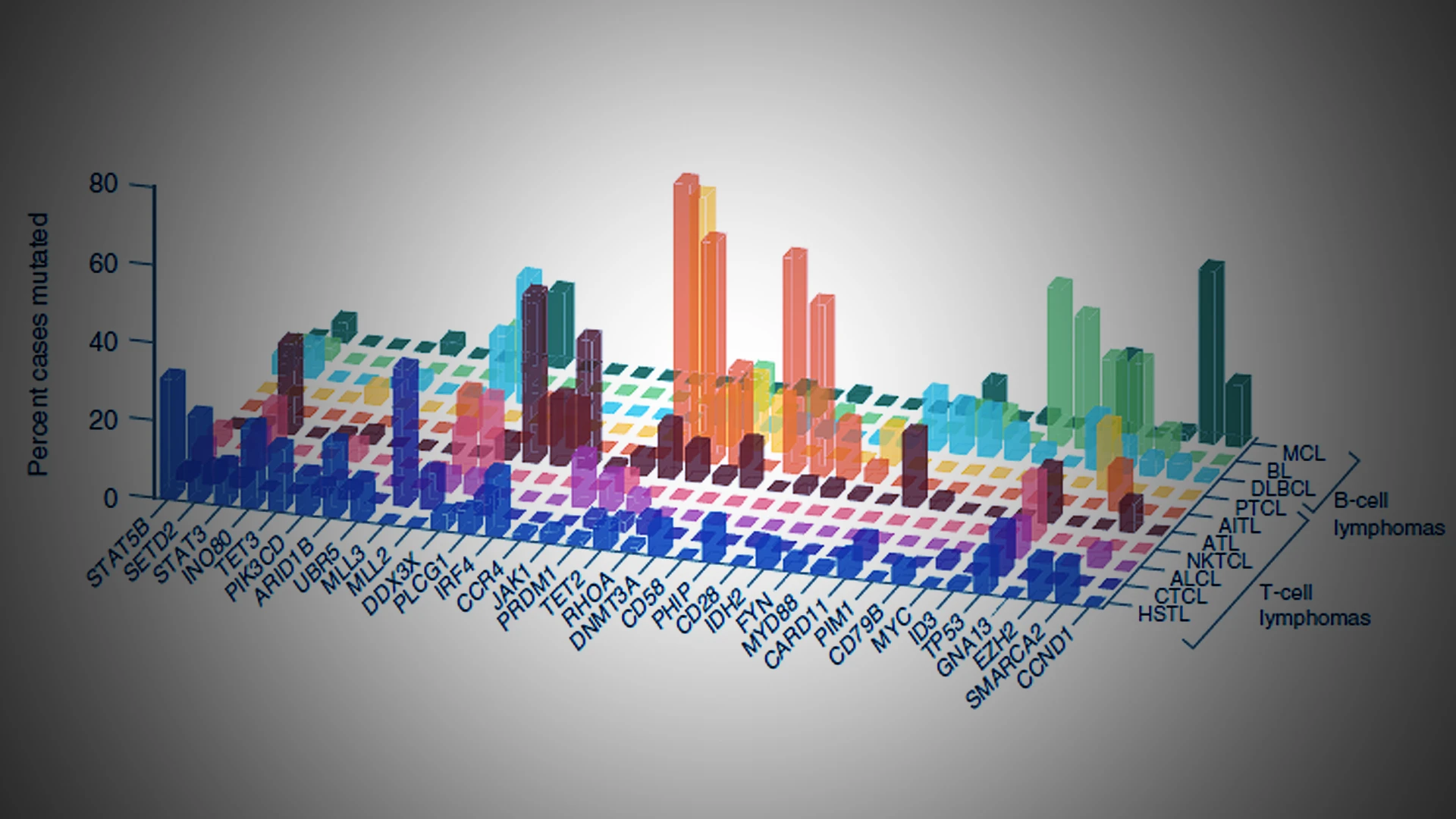
Specifically, they’re studying a gene that encodes INO80, a core component of the INO80c. This gene is often mutated in various cancers, including diffuse large B-cell lymphomas (DLBCL), hepatosplenic T-cell lymphoma (HSTL), some solid tumors, and in a subset of people with common variable immunodeficiency (CVID), a condition that increases the risk for lymphoma.
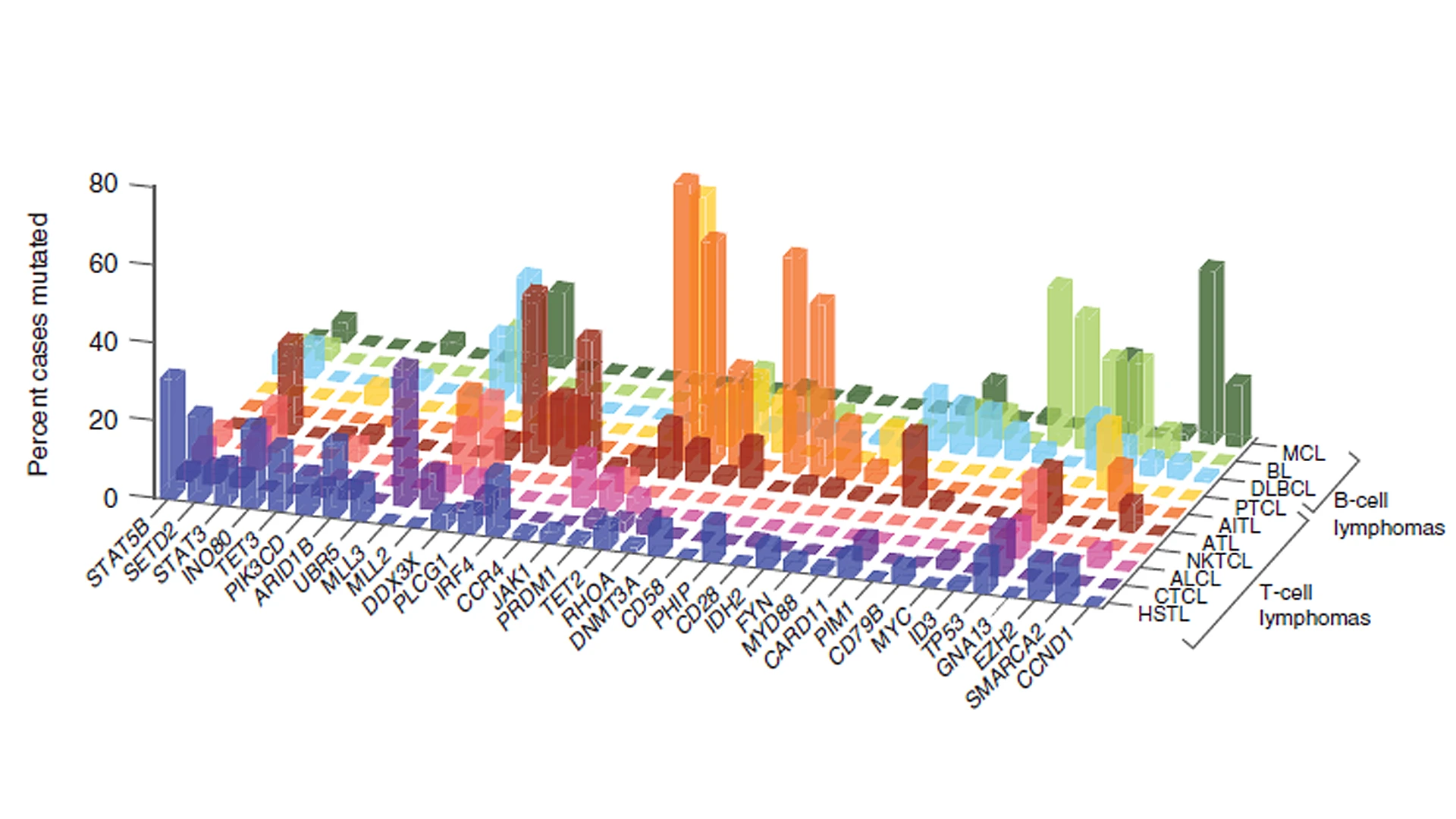
Hepatosplenic T-cell lymphomas (HSTLs) frequently manifest mutations in the genes SETD2, INO80, STAT5B, SMARCA2, TET3, and PIK3CD. In what is considered the largest genetic mutation landscape study of HSTL patients, a Mount Sinai researcher is working collaboratively to uncover the mechanistic workings of these mutations and implications for oncogenesis, proliferation, and tumor survival.
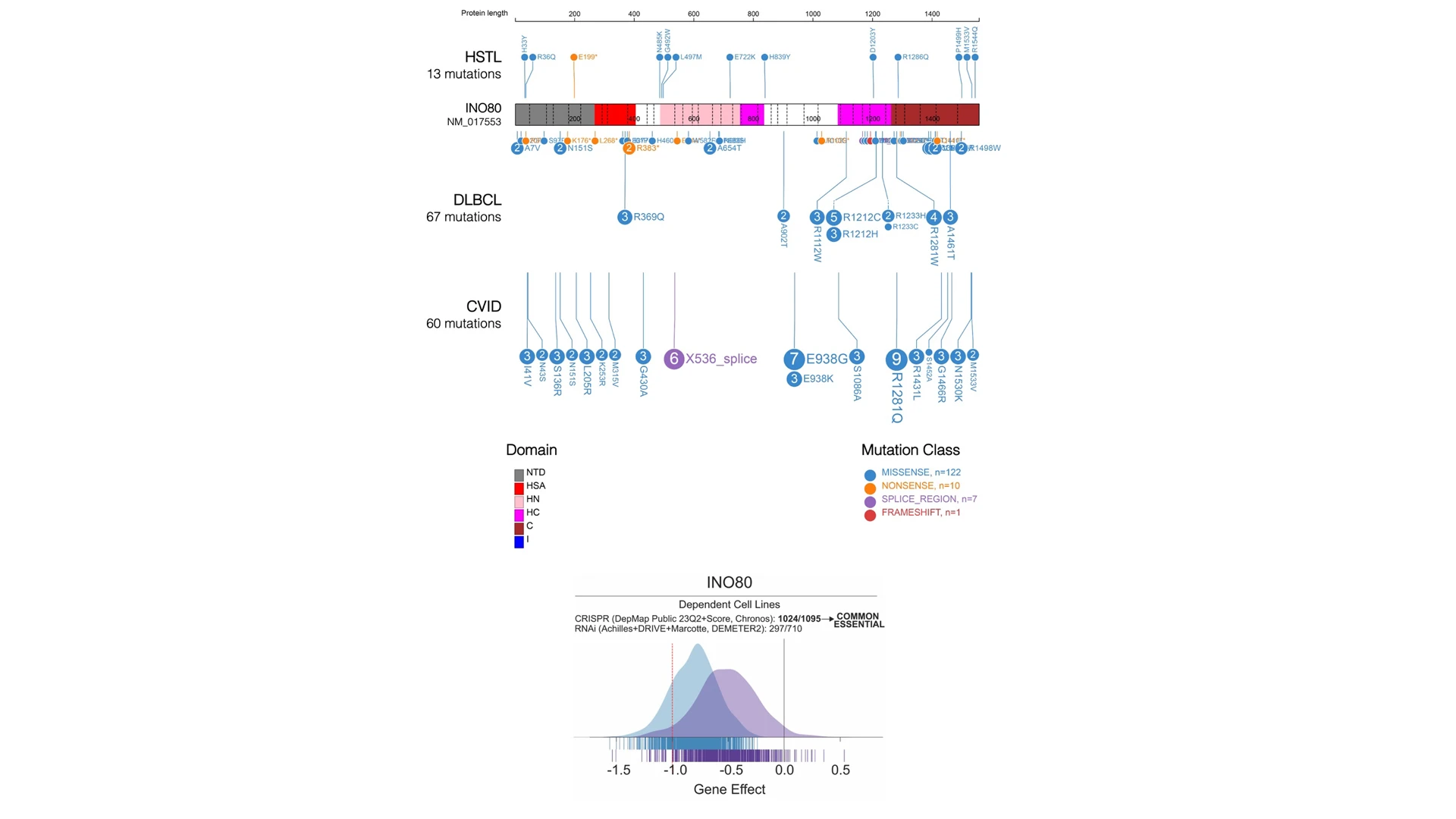
The research team is focusing on INO80 mutations as not much is known about the gene's role in immune cell dysfunction and cancer pathogenesis. The mutations are predicted to result in partial loss of function of the INO80c complex, which is considered essential for cell survival. The distinct INO80 mutations are a mixture of missense and nonsense mutations, with a small percentage of splice site mutations, some of which are predicted to result in terminally truncated proteins, pictured above. Although not identical, INO80 mutations in DLBCL, HSTL, and CVID overlap in distribution and pattern.
As all of the INO80 mutations appear to result in partial loss of function of the INO80c complex, enzymatic inhibitors of the mutant cells may represent an opportunity for targeted drug therapy in cancer. To even get to that stage, Dr. Dominguez-Sola and his team will first need to understand how these mutations contribute to disease pathogenesis and then create animal models for testing potential therapies.
“The interesting thing is that in lymphomas and other cancer types, there are common mutations in protein complexes that remodel chromatin, changing the chromatin environment essential to many cell processes. This has been known for quite a while,” says Dr. Dominguez-Sola. “But nobody has studied some of these mutations carefully, and so far not in cancer. We’re trying to model them and learn about their contributions.”
Previous studies examining various mutations in chromatin remodeling have mostly focused on their impact on gene expression. However, Dr. Dominguez-Sola’s team realized that the mutations’ impact are far more complex, and could be causing defective DNA repair or altering the metabolism of the cells, which is quite different from the prevailing view.
Validating this hypothesis opens up other possibilities about tumor pathogenesis and therapeutic intervention, adds Dr. Dominguez-Sola.
Collaborative Effort
Dr. Dominguez-Sola’s novel approach earned the team’s work a two-year $500,000 Mark Foundation–Gabrielle’s Angel Foundation Collaborative Grant in 2023, which is split between the Mount Sinai team and its collaborators at Rutgers Cancer Institute in New Jersey, under the leadership of Daniel Herranz-Benito, PharmD, PhD, Associate Professor of Pharmacology. This grant was designed to accelerate collaborations leading to potential cancer cures.
~21%
of HSTL cases feature INO80 somatic mutations
<10%-25%
5-year survival rates for HSTL patients
11 months
Median overall survival of HSTL patients
HSTL represents about 1.4% of T-cell lymphomas diagnosed worldwide
The collaboration came about because Dr. Dominguez-Sola’s lab had noticed the commonalities in the INO80 mutations found in CVID and B-cell lymphomas and wanted to learn more about function of this protein complex in B cells. This led to an outreach to Dr. Herranz-Benito, who said his group had looked at the same mutations in HSTL, a rare but deadly cancer, and the two groups launched a project to understand how these mutations work.
The combined team is exploring these areas simultaneously:
Categorizing functional loss of INO80 mutations and their equivalence across lymphoid disorders. The team is using functional complementation assays in vitro to provide structure-function correlates and help identify functional links among immune deficiencies and lymphomas.
Understanding INO80 dysfunction impacts on genomic maintenance and instability, metabolism, and lymphomagenesis by using INO80-haploinsufficient primary B and T cells to test the mechanistic effects of INO80 loss.
Modeling of INO80-mutant lymphoid malignancies and new preclinical models of HSTL. Via INO80-haploinsufficient mouse models combined with key gene mutations, the combined team aims to generate models of aggressive DLBCLs and HSTL. Currently, there are no models of HSTL accessible for research.
Thus far, their research suggests that INO80 mutations in lymphomas and immunodeficient patients seem to disrupt the protein's function in subtle ways, enough to cause disease but not to make the cells extremely sick, Dr. Dominguez-Sola explains.
“In fact, this is an essential protein. If we completely remove it from cells, they stop growing and die,” he says. Somehow, during cancer development, the tumor cells selected mutations that altered cell function enough to facilitate tumor formation, but were still compatible with growth and proliferation.
Therefore, it is conceivable that cancer cells with these mutations, such as in HSTL, would be more sensitive than normal cells to drugs that block INO80 function, since they already have reduced INO80 activity. These drugs do not exist yet, but a look at INO80's protein structure suggests that developing such therapies is possible, says Dr. Dominguez-Sola.
The research could see published results in about a year, especially for the HSTL model, Dr. Dominguez-Sola says. “Certainly, if the assay shows there is a metabolic defect and these mutations have been selected for that reason, that probably is something that we can explore quickly.”
Featured

David Dominguez-Sola, MD, PhD
Associate Professor of Oncological Sciences, and Pathology