Over the past decade, interest in normothermic machine perfusion (NMP) technology for sustaining organs outside the body has grown to new heights—especially so in liver transplantation, where there is a shortage. Using that technology, Mount Sinai researchers have found a new way to explore the metabolic landscape of hepatocellular carcinoma (HCC), a particularly aggressive type of liver cancer, and potentially transform the transplantation landscape.
Daniel Puleston, PhD, Assistant Professor of Immunology and Immunotherapy, and Oncological Sciences at the Icahn School of Medicine at Mount Sinai, has teamed up with M Zeeshan Akhtar, MBBCh, Assistant Professor of Surgery at the Icahn School of Medicine at Mount Sinai, as co-principal investigators.
The novel study, in its early stages, involves keeping HCC-laden livers alive outside the body using NMP, and exposing the livers to immunotherapy drugs and tracers, revealing the metabolic landscape of HCC before and after drug treatment.

Daniel Puleston, PhD, Assistant Professor of Immunology and Immunotherapy, and Oncological Sciences, is using perfusion technology in a novel way—keeping livers with tumors alive so that his team can examine how the cancer proliferates and evades immunotherapy. This high-risk, high-reward research could pave the way for better strategies for a treatment landscape that is fairly bleak.
In fact, Drs. Puleston and Akhtar have been awarded a Damon Runyon Cancer Research Foundation Award for their work, which supports high-risk, high-reward projects with the potential to significantly impact the prevention, diagnosis, and treatment of cancer.
What is the high risk? “The organs we’re working with are often barely functioning inside the body, and now we’re trying to keep them alive outside the body,” says Dr. Puleston. “Before we started out, there were no guarantees this would actually work.”
And what is the high reward? “We can now get insights into the human biology of cancer that no one has been able to achieve before,” he says.
A Practical Approach
Infusing metabolic tracers—heavy carbon versions of a nutrient-like glucose—to examine activity such as tumor metabolic flux and nutrient usage within cellular pathways has been around, but is impeded by considerable technical, logistical, and cost barriers, which is why the technique is so seldom employed in this country.
“Our labs’ practical approach to this challenge is to expose intact tumors within organs to metabolic tracers outside the body,” says Dr. Akhtar, an attending liver transplant surgeon and scientist who is a leading expert in normative machine perfusion.
“These tools allow us to trace the flow of heavy atoms throughout the metabolome with mass spectrometry, providing the opportunity to assess in situ cellular metabolism on a regular basis in human tissues and cancers,” he adds.
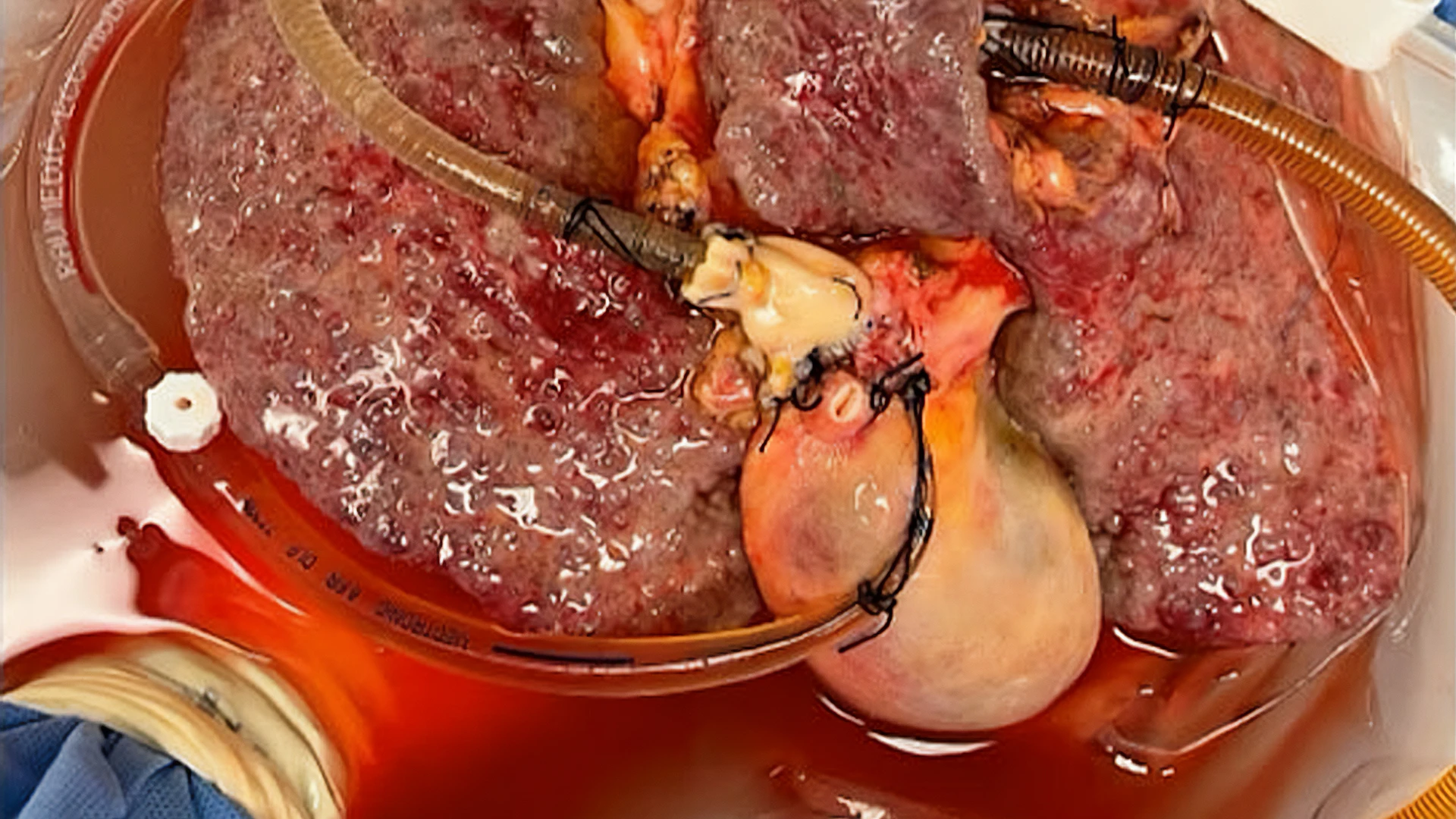
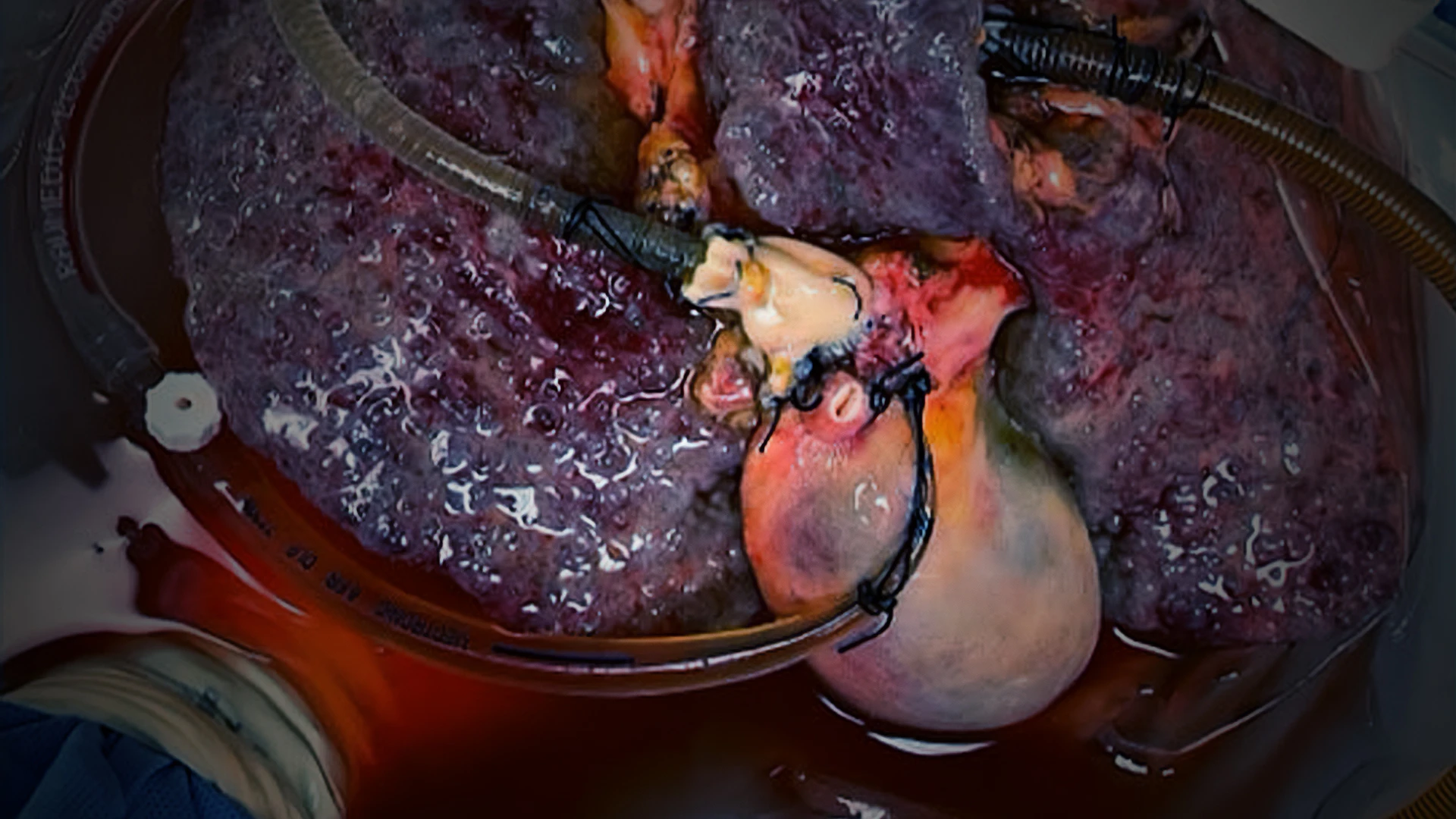
Using normothermic machine perfusion, the Mount Sinai team is able to keep intact, tumor-bearing organs alive outside of the body. This allows the researchers to investigate the biology and metabolism of cancer and immune cells while they reside within their native tumor environment.
This method allows Drs. Puleston and Akhtar to experimentally manipulate those tissues and tumors in a way that is quite unique and beneficial. An offshoot of this research has resulted in the team prioritizing a project to genetically re-engineer and improve an intact human organ perfused through NMP so it is suitable for transplantation in patients with HCC.
“We currently discard many donor livers because they aren’t quite healthy enough for transplantation,” says Dr. Puleston. “If we could take these sub-optimal organs and improve them by manipulating their tissue in ways that are clinically and scientifically unique and acceptable, then we could potentially help bridge the gap in this country and elsewhere between supply and demand of viable organs.”
Improving a Bleak Landscape
The stakes are particularly great around HCC, the second most lethal form of cancer after pancreatic. Surgical removal of the tumor or a liver transplant may be necessary to treat the most advanced cases, though the national shortage of human livers for transplantation has resulted in 15 to 20 percent of patients expiring while on the recipient waiting list.
Drs. Puleston and Akhtar are directly addressing this urgent problem by investigating the potential of improving allografts deemed clinically marginal due to the age, health status, or other complicating factors of the donor.
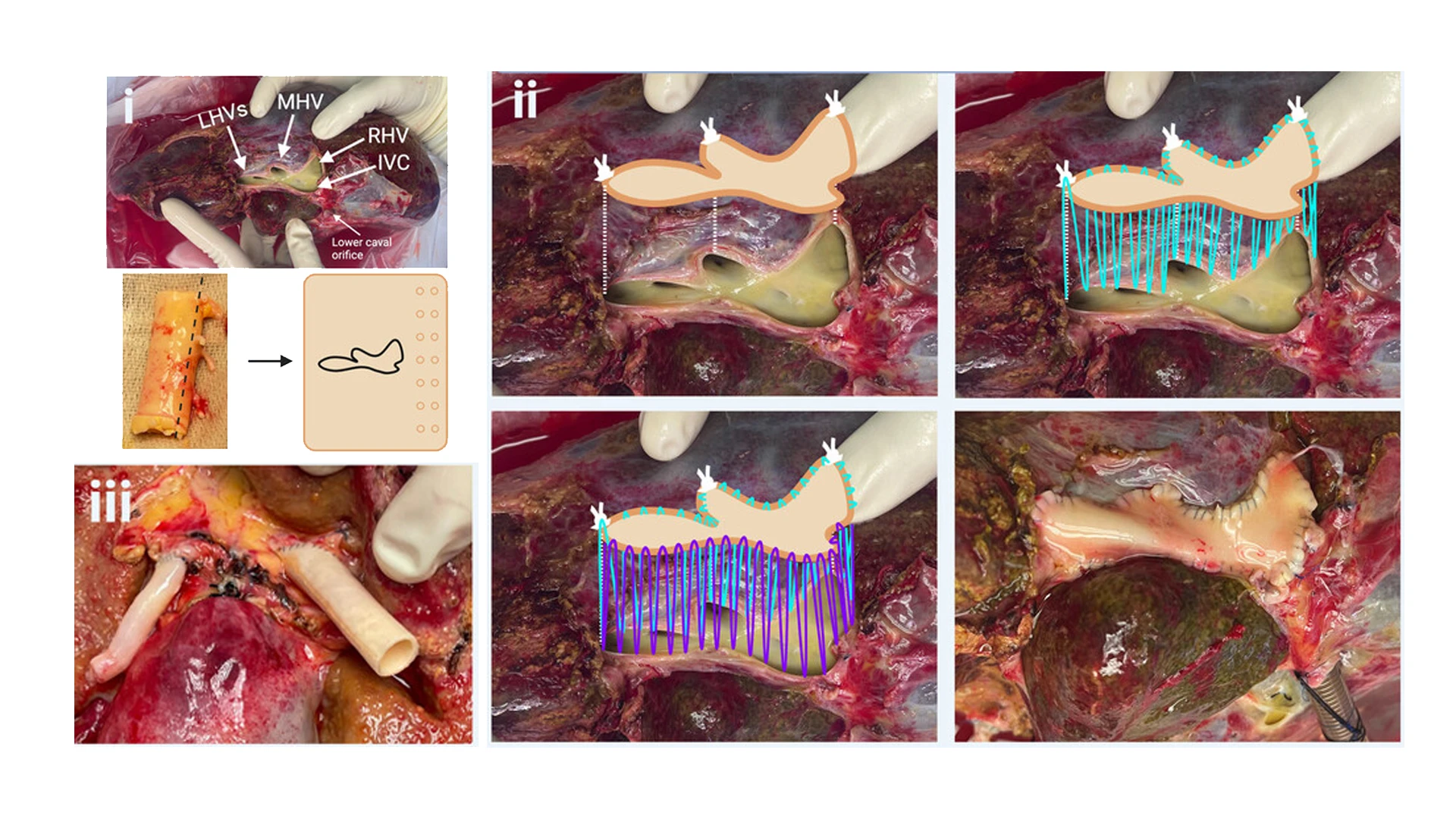
In a related study published on Artificial Organs, the research team found validation for using closed-circuit normothermic machine perfusion as a platform to study organs for mechanisms of disease and applied therapeutics. The technique involved reconstructing a singular venous outflow tract to make the hepatectomized livers compatible with the perfusion machine, by suturing a graft covering various hepatic veins and the inferior vena cava (images I through III).
The hope is that, collectively, the findings of tumor biology and creating a more robust supply of viable organs for transplant can also improve existing therapeutics or lead to the discovery of new ones.
“Through our unique approach, we can begin to integrate metabolism with gene expression, epigenomics, and protein data to build a comprehensive picture of how cells interact and are regulated in HCC,” explains Dr. Puleston. “We expect these data to reveal new nodes for immune and tumor regulation, as well as potential metabolic vulnerabilities that can be exploited for therapeutic benefit.”
Promising targets include immune checkpoint inhibitors (ICIs), a breakthrough class of cancer drugs that has been used in combination with other treatments, or standalone. As a single agent, ICIs could have a clinical response rate of about 15 to 20 percent. Researching why ICIs don’t work, however, has often been restricted to biopsies following tumor resection after the treatment course has finished, which precludes the early events that occur when an ICI agent first enters a tumor.
“We’re confident that by using NMP on hepatocellular cancerous livers, we will be able to understand key events that play out within tumors in the early hours and days upon exposure to ICIs,” notes Dr. Puleston. His hope is that this unique approach will answer several key questions, including how ICIs function mechanistically within tumors, what specific cells are being bound by therapeutic ICI antibodies, and what are the initial biologic changes and signaling events driven by ICI antibody infusion.
“Our findings could well provide better insights into how to optimize these agents so more patients respond,” emphasizes Dr. Puleston. “And on a much broader scale, we feel this type of research, where we probe the action of drugs in ways not previously possible, could offer an exciting new platform for scientific research across a wide range of cancers in humans.”
Featured

Daniel James Puleston, PhD
Assistant Professor of Immunology and Immunotherapy, and Oncological Sciences

M Zeeshan Akhtar, MBBCh
Assistant Professor of Surgery