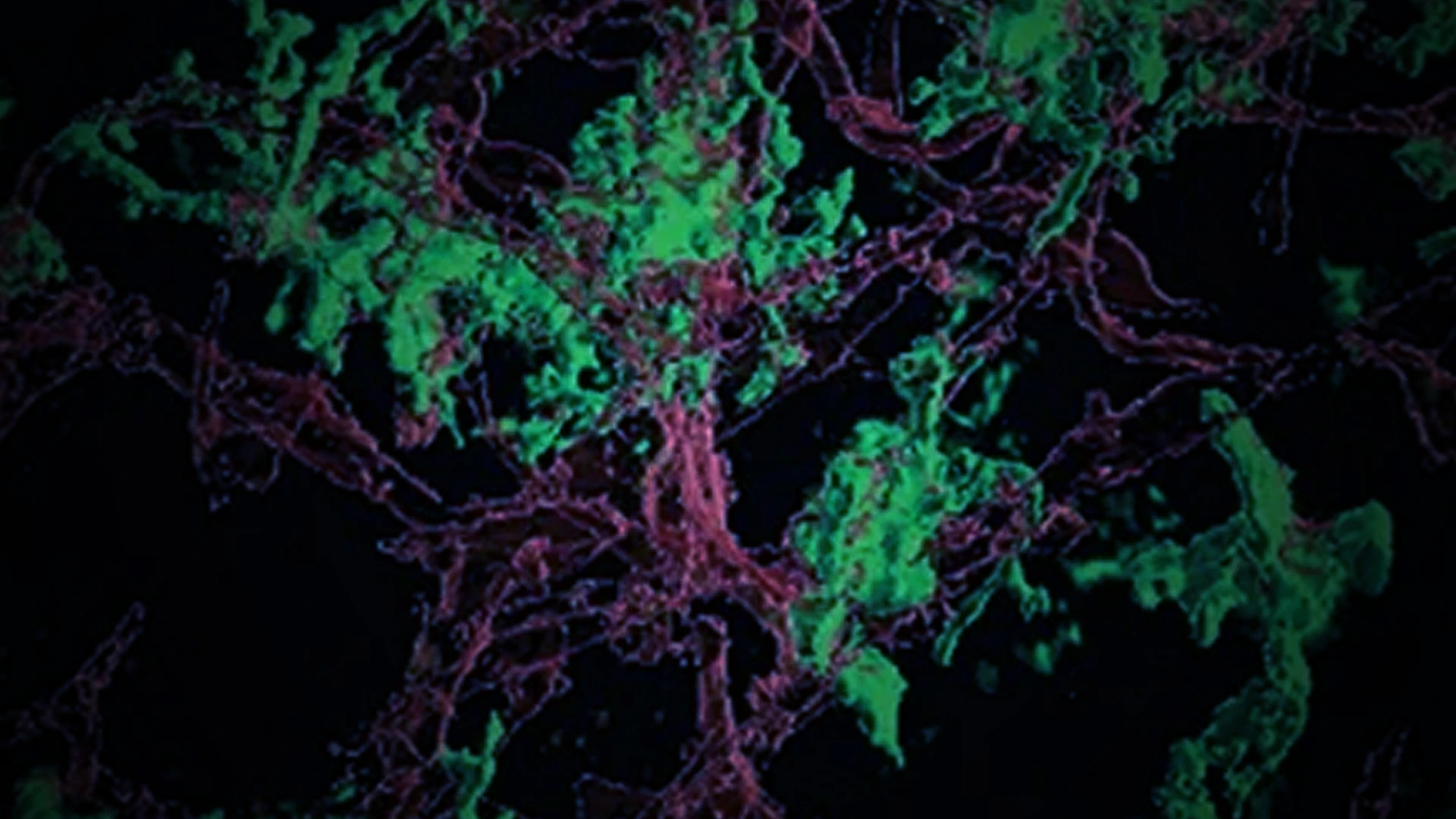
Researchers at the Icahn School of Medicine at Mount Sinai are zeroing in on a new target in the quest to defeat glioblastoma, an aggressive type of brain cancer. Project leads Dolores Hambardzumyan, PhD, MBA, Professor of Oncological Sciences, and Neurosurgery, and Hongyan Zou, MD, PhD, Professor of Neuroscience, and Neurosurgery, received an R01 grant from the National Institute of Neurological Disorders and Stroke in September 2023. The five-year, $3.43 million grant will support their investigation into the role of microglia, resident immune cells in the brain that congregate at the margins of glioblastoma cells, in glioblastoma invasion and relapse.
Glioblastoma is among the deadliest cancers, with an average survival rate of just 15 months. It is almost certain to recur after surgery, usually within six months. “The cells are very invasive, easily intruding into healthy areas of the brain,” Dr. Hambardzumyan says. Those invasive cells hide in nearby tissues, making it virtually impossible to resect the tumor completely. “That’s why, when glioblastoma recurs, it’s usually within 2 to 3 centimeters of the surgical cavity,” she says.
Given this deadly pattern, Dr. Hambardzumyan says it is critical to understand what’s happening on the margins of glioblastoma tumors.
Microglia’s Role in Glioblastoma
Microglia, which are native to the brain, and bone marrow-derived macrophages are both abundant in the glioblastoma microenvironment. Historically, though, the two types of cells in the brain were indistinguishable from one another. Researchers often lumped the blood-derived cells and brain-derived cells together under the umbrella of “tumor-associated myeloid cells.” But Dr. Hambardzumyan found that the two cell types behave quite differently.
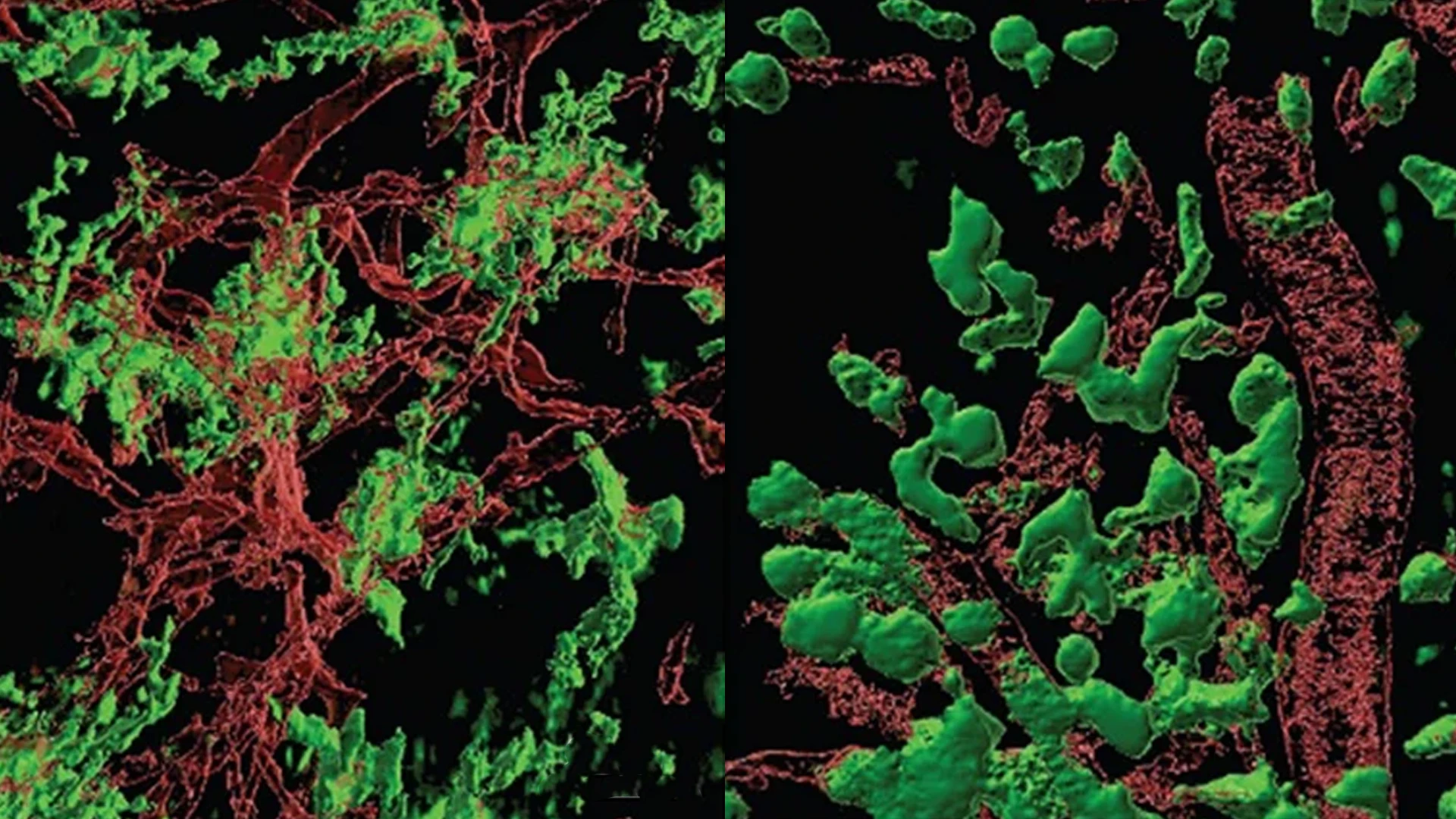
The most abundant cells in the tumor microenvironment of glioblastoma are tumor-associated macrophages, comprising both brain-resident microglia and bone marrow-derived macrophages. Previously, the two were considered indistinguishable, but through the work of Dolores Hambardzumyan, PhD, MBA, and Hongyan Zou, MD, PhD, they were able to demonstrate microglia (left) appeared and behaved differently from bone marrow-derived macrophages (right).
Much of her previous research focused on identifying markers to distinguish the blood-derived cells from native microglia. “Once we could discriminate between them, we found the cells that come from the blood are located within the tumor itself. But the microglia get pushed to the tumor borders,” Dr. Hambardzumyan says. “They form a ring around the tumor.”
That ring of microglia interacts with tumor cells to form a pro-tumorigenic loop that drives disease progression. “They form this vicious cycle of interdependency that’s very difficult to treat,” Dr. Hambardzumyan explains. That process, she and Dr. Zou believe, creates an invasive edge around the tumor that allows it to infiltrate nearby cells. “By breaking that interdependency between the microglia and the tumor cells, we hope to sensitize the cells to therapies and decrease the recurrence rate,” she adds.

Dr. Zou (left) and Dr. Hambardzumyan (right) have discovered that microglia congregate preferentially in a ring at the margins of glioblastoma. They are delving into how this ring performs signaling, which they could in turn exploit to block proliferation and block invasion.
Using mouse models of human glioblastoma, the researchers are targeting that invasive ring with a one-two punch. Dr. Hambardzumyan’s research identified an interleukin-1 signaling pathway that drives microglia proliferation. Dr. Zou’s work highlighted the role of plexin-B2, an axon guidance receptor important for microglia alignment and invasion. “We both had this promising preliminary data, so we decided to combine forces using both targets,” Dr. Hambardzumyan says. “We want to block proliferation and block invasion to paralyze microglia on the edge, so they can’t help tumors facilitate cell invasion at the border.”
Molecules targeting interleukin-1 and plexin-B2 signaling pathways already exist, and the U.S. Food and Drug Administration has approved some of those compounds for use in other diseases. Now the researchers are testing the combination by injecting the drugs directly into tumor cells in the mouse brain. Glioblastoma tumors are notoriously heterogeneous, so the research team will test the approach in multiple genetic models of the disease. “If we can show these are beneficial in various genetic landscapes, I’m hopeful we can move into human clinical trials for recurrent glioblastoma even before the end of this five-year grant period,” Dr. Hambardzumyan says.
Team Science
Dr. Hambardzumyan moved her lab to Icahn Mount Sinai four years ago because of the strength of its neuro-oncology program. That decision, she says, has enabled her to partner with other experts to accelerate the pace of glioblastoma research. “The researchers at Mount Sinai go out of their way to work as a team for better outcomes,” she says.

Drs. Zou (left) and Hambardzumyan (right) are collaborating with other specialists at Mount Sinai, from the departments of Neuroscience, and Neurosurgery, to devise actionable clinical strategies. They are optimistic they will find novel pathways in altering the trajectory of glioblastoma, which has historically poor survival rates.
By combining her expertise in microglia and mouse modeling with Dr. Zou’s deep knowledge of plexin-B2 and the clinical aspects of glioblastoma, they and their colleagues are optimistic they can make progress against this formidable cancer. The study also includes Icahn Mount Sinai co-investigators Raymund Yong, MD, Associate Professor of Neurosurgery; Alexander Tsankov, PhD, Assistant Professor of Genetics and Genomic Sciences; and Roland Friedel, PhD, Associate Professor of Neuroscience, and Neurosurgery.
“This is a complex disease that requires a team effort,” Dr. Hambardzumyan says. “When you combine forces, that’s where the real science is.”
Featured

Dolores Hambardzumyan, PhD, MBA
Professor of Oncological Sciences, and Neurosurgery

Hongyan Zou, MD, PhD
Professor of Neuroscience, and Neurosurgery