Hematopoietic stem cell or bone marrow transplantation is a lifesaving procedure, but it can result in life-threatening complications, such as graft-versus-host disease (GvHD), in which the transplanted cells regard the recipient body as foreign and attack it.
Mount Sinai has received continuous grant funding for the past 35 years from the National Cancer Institute (NCI) to study the condition, most recently a $13 million renewal in 2022. This renewal is powering a pair of cutting-edge clinical trials that could reshape future research and therapeutic strategies around a disease that affects roughly half of all patients with hematologic malignancies treated with allogeneic hematopoietic stem cell transplantation.

James Ferrara, MD (left), and research associate Rahnuma Beheshti (right) analyzing biomarkers that measure gastrointestinal tissue damage from a blood sample of a patient participating in a Mount Sinai sponsored clinical trial.
“We didn’t expect to see this kind of rapid advance in a field where clinical trials are extremely difficult and where no drugs at all existed until just several years ago,” says James Ferrara, MD, Professor of Medicine (Hematology and Medical Oncology), and Oncological Sciences at the Icahn School of Medicine at Mount Sinai. The newest grant renewal, which provides funding for five years, underscores the evolutionary progression of the team’s GVHD research from basic science to translational models to clinical trials, bringing the field closer to the goal of transformative therapeutic strategies for GvHD patients, says Dr. Ferrara, who has served as Principal Investigator of the NCI grant since 1997.
Reaching Countless Patients
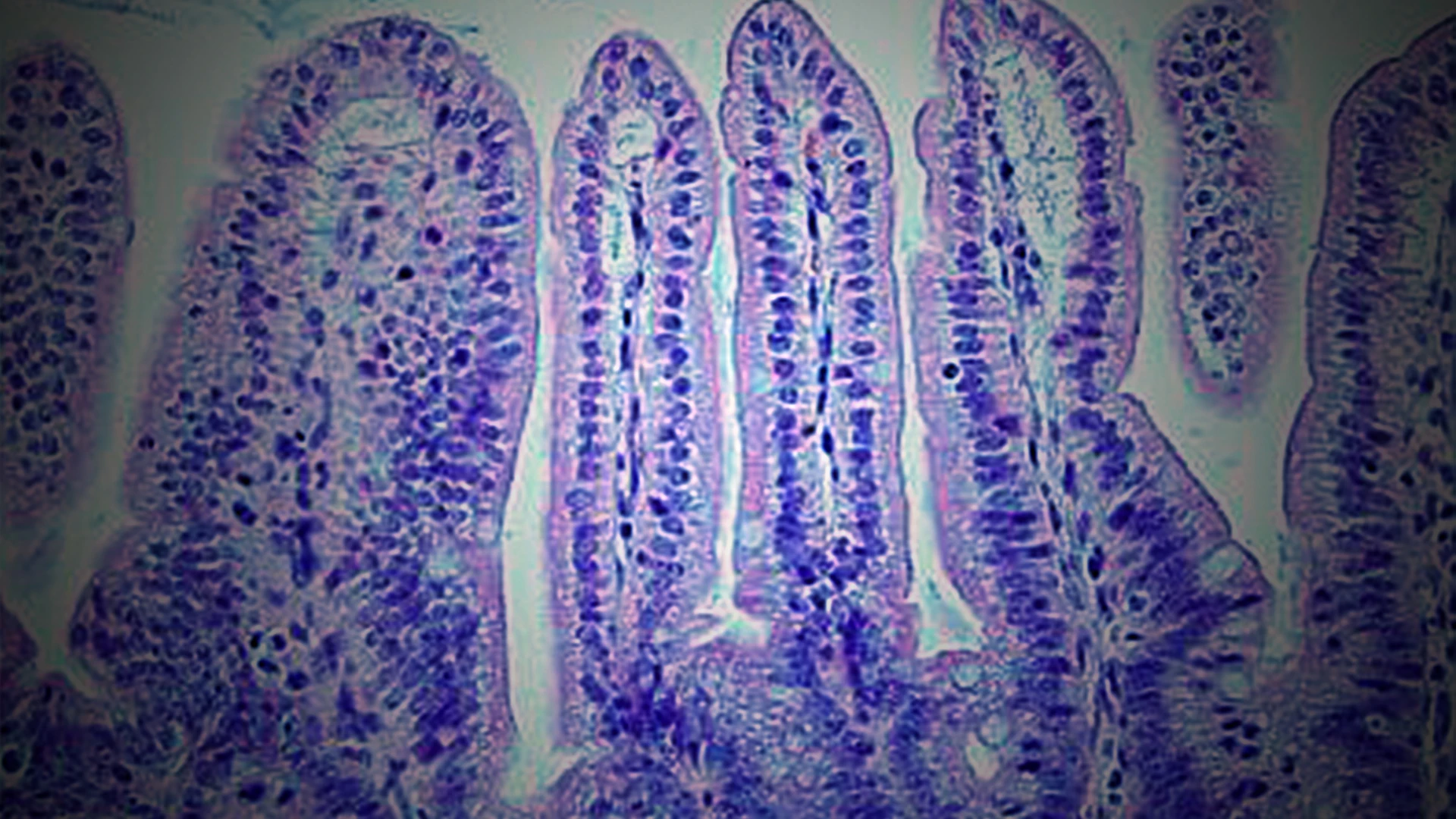
Key lines of research from the Ferrara Bone Marrow Transplantation Laboratory have included serum biomarkers that help stratify risk for patients, and the presentation of the disease in the gastrointestinal (GI) tract—the organ that is most resistant to standard steroid treatment and accounts for the vast majority of non-relapse mortality.
The lab is working on biomarkers that accurately measure GI tissue damage to intestinal crypts, on regenerating cells within the GI tract, and a method to stratify GvHD patients into high- and low-risk groups.

Dr. Ferrara (back) and Alberto Utrero-Rico, PhD (front), are advancing work that has received continuous funding from the National Cancer Institute for 35 years.

Dr. Ferrara (center) with team members Mariano Acosta Prado, PhD (left), and lab manager George Morales (right). Several clinical trials are underway, conducted as part of the Mount Sinai Acute GVHD International Consortium (MAGIC).
“These biomarkers have become the industry standard for how we investigate new therapeutic agents and have allowed us to accelerate our research and start conducting clinical trials in patients,” Dr. Ferrara notes.
The serum protein-based biomarker technology from Dr. Ferrara’s lab has been licensed to Eurofins Viracor, which offers a commercial test used by more than 100 hematopoietic stem cell transplant centers across the country. This test is used to predict which patients are most likely to respond to standard corticosteroid therapy and benefits more than a thousand GVHD patients annually.
New Approaches
Dr. Ferrara’s team is exploring new pathways and strategies in GVHD, and is embarking on two clinical trials.

The team, from left to right: Alberto Utrero-Rico, PhD; George Morales; James Ferrara, MD; Rahnuma Beheshti; Mariano Prado Acosta, PhD; and Steven Kowalyk.
The first trial is underway and explores leveraging receptor-interacting protein kinase (RIPK1) enzymes via a newly discovered pathway with known links to destroying critical stem cells in the GI tract. The enzyme has roles in controlling inflammatory cell death, and the trial, testing a drug from Genentech that acts as a RIPK1 inhibitor, could potentially promote crypt regeneration.
“This first-in-human trial has already treated two patients who have responded very well to the therapy in combination with standard systemic corticosteroids, leaving us very excited about its prospects,” says Dr. Ferrara, who is leading this Genentech-sponsored study.
The second trial, which is awaiting approval from the U.S. Food and Drug Administration, aims to reduce the use of potentially harmful corticosteroids. These drugs are routinely given to GVHD patients of all risk levels, and as a result, some low-risk patients are overtreated—suffering steroid toxicity—while some high-risk patients are undertreated and have their conditions poorly managed. Dr. Ferrara’s serum biomarkers are enabling researchers to monitor in real time and identify patients with minimal crypt damage who could be safely treated at much lower doses of steroids.
These trials are part of a global collaborative effort known as Mount Sinai Acute GVHD International Consortium (MAGIC). Formed nine years ago when Dr. Ferrara moved his lab to Mount Sinai from the University of Michigan, MAGIC is committed to finding therapeutic solutions to GVHD with the help of 24 major research centers in the United States and worldwide.
“One of our strengths is being able to collectively monitor almost 700 patients a year, allowing us to validate our findings much more quickly,” says John Levine, MD, Professor of Medicine (Hematology and Medical Oncology), and Pediatrics at Icahn Mount Sinai, who trained under Dr. Ferrara at the University of Michigan and now co-directs MAGIC with him.
“Other centers were anxious to collaborate with us to study GVHD and conduct clinical trials, and the collective progress we’ve made has been amazing,” says Dr. Levine. “We’re convinced in the next five years this effort will lead to multiple new drugs and the elimination of steroids in treating a significant number of patients with GVHD.”
Featured

James Ferrara, MD
Professor of Medicine (Hematology and Medical Oncology), and Oncological Sciences