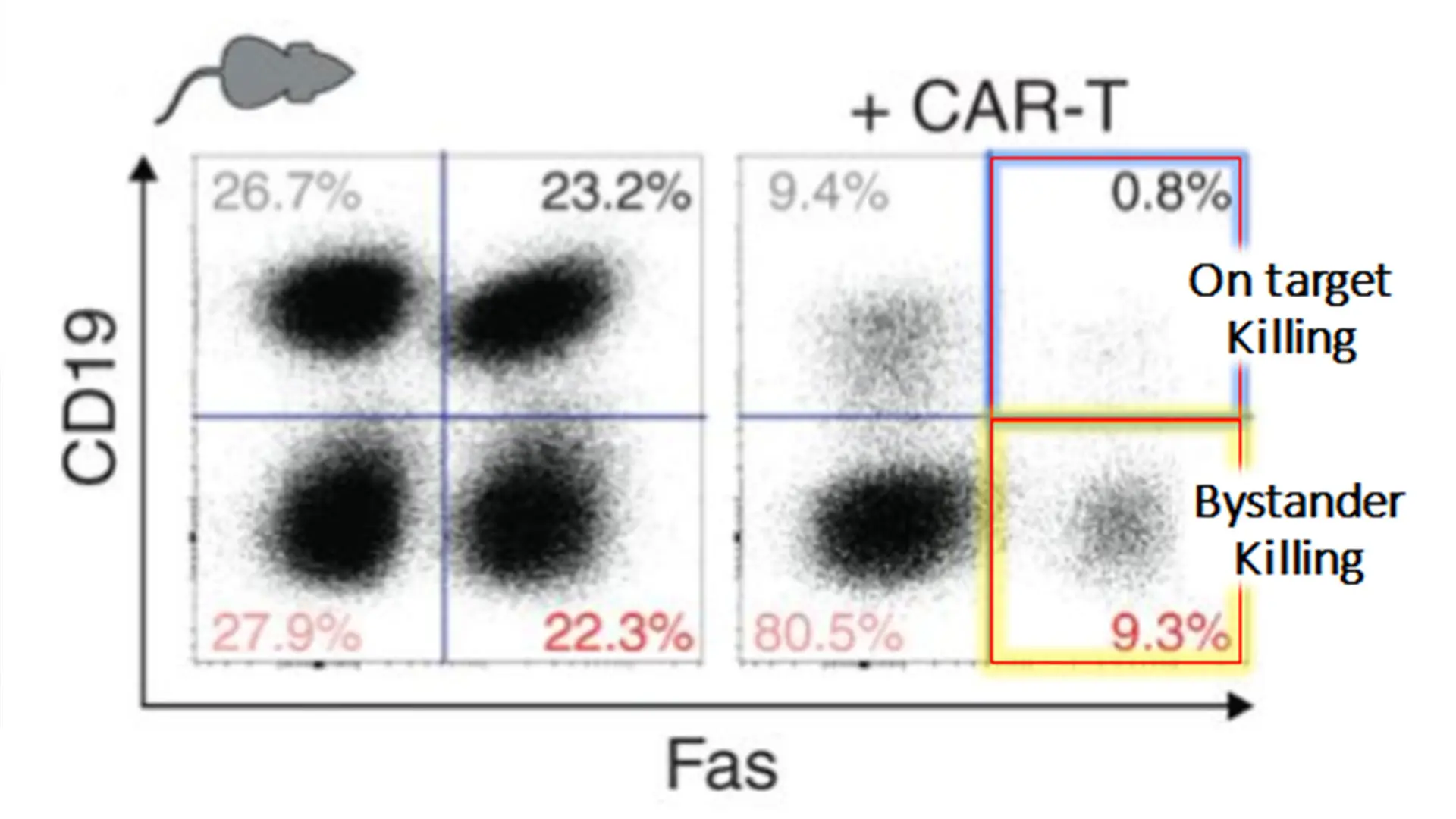
Mixtures of CRISPR gene-edited murine lymphoma cells were treated with murine anti-CD19 CAR-T cells, demonstrating that both "On Target" and "Bystander" lymphoma killing is highly fas-dependent. Patients with high fas-expressing tumors were more likely to have durable remissions.
One major limitation of T-cell-based immunotherapies is a phenomenon known as “antigen escape,” which occurs when a subset of cancer cells lose the targeted antigen. Patients’ tumor regressions will stall and they will relapse quickly when even one cancerous cell is able to evade detection by CAR-T, anti-PD1, or bispecific antibody immunotherapies.
At The Tisch Cancer Institute, researchers led by Joshua Brody, MD, Associate Professor of Medicine (Hematology and Medical Oncology) and Oncological Sciences, are working to prevent antigen escape by boosting a process called “bystander killing,” where targeted tumor cells are destroyed along with bystander tumor cells, regardless of whether they express an antigen. Dr. Brody described Mount Sinai’s study of Fas-mediated, antigen-specific T-cell killing in the March 2021 issue of Cancer Discovery.
“We now understand how important the Fas mechanism is—consisting of FasL on the T cell activating Fas on the tumor cell—for on-target killing of cells, as well as for destruction of off-target, or bystander cells,” says Dr. Brody, the study’s senior author. “Our research describes for the first time in vivo Fas-dependent bystander killing of antigen-negative tumors by T cells.” Those results, he adds, suggest that levels of Fas may be contributing to sensitivity to off-target killing and that FasL expression of T cells is also being modulated and may play a key role in Fas-mediated apoptosis.
Mount Sinai's approach not only treats but prevents antigen escape.
In the case of CAR-T and bispecifics, the loss of targets such as CD19 or CD20 proteins expressed on the tumor surface provide the escape mechanism. For anti-PD1, the specific antigens may be unknown, but they are generally presented on major histocompatibility complex (MHC-1) molecules, so MHC-1 loss is a common mechanism of antigen escape for patients treated with anti-PD1 antibodies.
A significant portion of research has been devoted to finding ways to treat antigen-escaping tumors after the problem occurs. But
the Mount Sinai team focused on preventing antigen escape through bystander killing mediated by the FasL-Fas pathway. They
discovered that certain regulators of Fas signaling can be inhibited with small molecule therapies that can increase the efficacy of bystander killing and help to prevent patient relapse.
“Some of these therapies have been approved by the [U.S. Food and Drug Administration] FDA and are already in the clinic, while others are undergoing trials,” Dr. Brody says. “The beauty of all
of them is that they may impact not only on-target killing of cancer cells, but that they’re uniquely able to eliminate the one remaining antigen-negative cell—the straggler—that manages to escape immunotherapy. We hope to incorporate these agents into upcoming clinical trials.”
"We hope to incorporate these agents into clinical trials."
The Mount Sinai researchers also made another important finding, which could provide physicians with a potentially valuable biomarker and demonstrates the importance of these molecular pathways in patients: tumoral expression of Fas seemed to predict survival in CAR-T treated patients.
“What this team has achieved is a highly practical approach to preventing rather than just treating antigen escape,” Dr. Brody says. “And that represents significant progress for the field in terms of better understanding and addressing the central problem of all current immunotherapies, which affect about one-third of cancer patients.”
Featured

Joshua Brody, MD
Associate Professor of Medicine (Hematology and Medical Oncology), and Oncological Sciences
