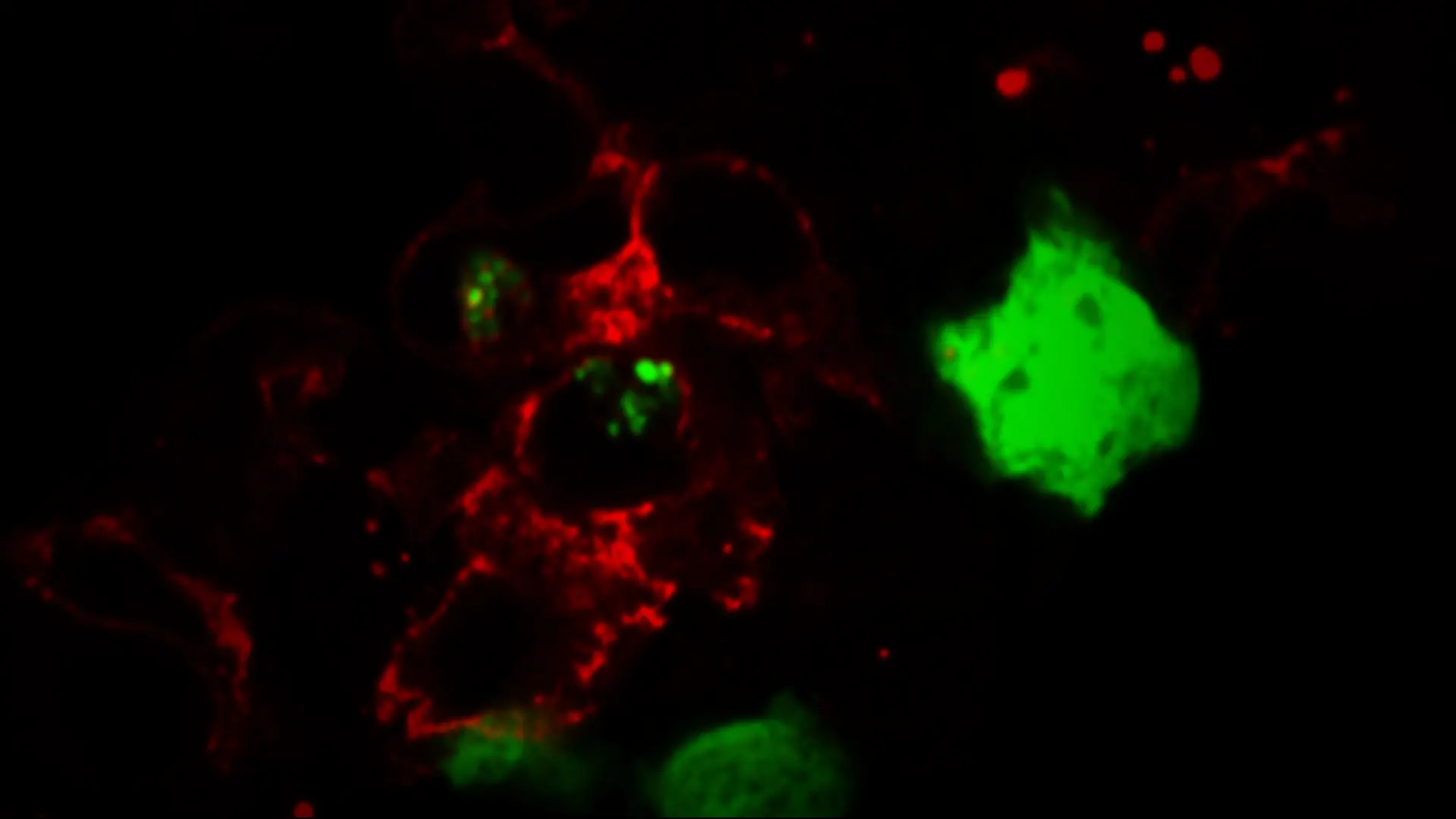
Tissue resident macrophages, in red, are shown eating tumor cells, in green.
For the first time, Miriam Merad, MD, PhD, Director of the Precision Immunology Institute, and her research team at the Icahn School of Medicine at Mount Sinai have shown that specific populations of macrophages promote tumor growth and contribute to immune evasion in early lung cancer lesions. Their findings, which appeared in the June 21, 2021, issue of Nature, used a novel genetic approach to trace the lineage of macrophages in non-small cell lung carcinoma.
Macrophages—specialized immune cells involved in homeostasis and tissue repair—exist in every organ of the body. They also have a key role in shaping the tumor microenvironment (the cells, molecules, and tissues surrounding the tumor), tumor immunity, and the patient’s response to immunotherapy, making them an important target for cancer treatment. However, the ability to develop strategies to modulate macrophages has been hampered by an incomplete understanding of their molecular and functional diversity in the tumor setting.
In earlier work, Dr. Merad, who is also Professor of Oncological Sciences, and Medicine (Hematology and Medical Oncology), discovered that macrophages arise from two distinct origins: self-renewing tissue-resident macrophages (TRMs), which are established during embryonic development and persist into adulthood, and short-lived monocyte-derived macrophages (MDMs), circulating white blood cells that accumulate mostly in inflamed lesions.
“Looking at the changes in the macrophage populations over time with a great level of precision helps us to understand where and when we might be able to intervene most effectively to prevent the tumor from advancing.”
-Miriam Merad, MD, PhD
In the recent study, Dr. Merad and her team used a combination of single-cell RNA sequencing to capture the cells’ heterogeneity and “fate mapping” (labeling and imaging of genes over time) to trace their developmental history. They found that TRMs and MDMs in both humans and mice contribute to lung tumor formation and growth in distinctly different ways with distinct spatial and temporal distribution in the tumor microenvironment.
The biology is extremely complex, says Dr. Merad. TRMs accumulate close to, and interact with, tumor cells in the very early stages of disease and begin a “repair program,” which, through a series of transcriptional and epigenetic changes promotes tumor invasiveness and growth. At the same time, and in an effort to promote repair, TRMs promote a strong T regulatory cell response that shuts down T-cell recognition and elimination of tumor cells, further enabling tumor growth.
To test the role for TRM in tumor growth, the researchers depleted the TRMs prior to tumor implantation, which dramatically reduced invasiveness and halted growth. Depleting TRMs at later stages of disease, however, appeared to have no benefit.
Depleting TRMs prior to tumor implantation reduced invasiveness.
These results suggest that modulation of TRM in patients at risk for developing lung
cancer could significantly halt lung cancer development. “Looking at the changes in the macrophage populations over time with a great level of precision helps us to understand where and when we might be able to intervene most effectively to prevent the tumor from advancing,” says Dr. Merad.
Lung cancer is the leading cause of cancer mortality in the United States, and more
than 60 percent of patients are diagnosed with late-stage disease. Dr. Merad says Mount Sinai’s early lung cancer screening program creates an opportunity to catch and target lesions in earlier stages when they are more treatable.
“Tissue resident macrophages play critical homeostatic roles in all of the tissues in which they reside and represent a major untapped source of therapeutic targets for many human diseases,” she adds.
The research team is now applying the sophisticated genetic tools and analyses developed
in the latest lung cancer study to assess TRMs in early lesions in liver cancer, soon to be followed by studies in colon cancer.
Featured

Miriam Merad, MD, PhD
Director of the Precision Immunology Institute