About half of all patients with muscle-invasive urothelial carcinoma will experience metastatic recurrence after chemotherapy and surgical removal of the bladder. But their odds may improve now that the U.S. Food and Drug Administration (FDA) has approved the adjuvant use of an immune checkpoint inhibitor—nivolumab—based on research from the Icahn School of Medicine at Mount Sinai, published June 3, 2021, in The New England Journal of Medicine.
Matthew Galsky, MD, Professor of Medicine (Hematology and Medical Oncology), and Urology at The Tisch Cancer Institute, co-led the study. Dr. Galsky has focused on making immunotherapy work for patients with urothelial cancer for more than a decade. During a Phase lll trial, the adjuvant use of nivolumab was evaluated in more than 700 patients who were randomized at 156 sites in 29 countries.
The results showed that patients who received nivolumab (240 mg) had a median disease-free survival rate of 20.8 months, compared with 10.8 months for those receiving a placebo. In addition, 74.5 percent of patients given nivolumab who had a programmed death ligand (PD-L1) expression of 1 percent or higher were disease-free at six-months, vs. 55.7 percent of those who received a placebo. Treatment-related adverse effects of grade 3 or higher occurred in less than 18 percent of patients, compared with 8 percent of those receiving placebo. The protein PD-L1 is a potential biomarker to identify patients who might benefit from nivolumab.
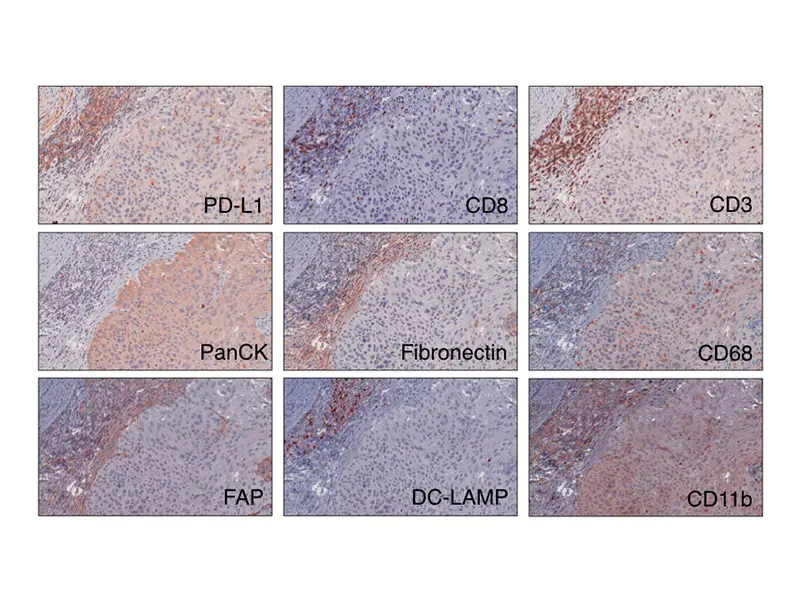
This analysis of the cellular composition of the bladder tumor microenvironment uses multiple immunohistochemical stains on a single slide to dissect the features associated with response and resistance to immune checkpoint blockade.
This is a milestone for patients who have undergone major surgery to remove their bladder or parts of the urinary tract but are still in need of additional therapeutic approaches that can reduce the risk of their urothelial carcinoma returning.
- Matthew Galsky, MD
“Our study is the first to show a benefit from immune checkpoint blockade in the adjuvant setting for muscle-invasive urothelial cancer,” says Dr. Galsky, the study’s senior author, and Associate Director for Translational Research at The Tisch Cancer Institute. “That’s why it represents a major milestone for patients who have undergone major surgery to remove their bladder or parts of the urinary tract but are still in need of additional therapeutic approaches that can reduce the risk of their urothelial carcinoma returning.”
Urothelial cancer accounts for more than 70,000 new cases and roughly 15,000 deaths each year in the United States. The standard of care for tumors originating in the bladder has been neo-adjuvant chemotherapy prior to radical surgery. Unfortunately, microscopic metastatic cancer cells are often left behind, increasing the risk of cancer recurrence.
Nivolumab is a fully human IgG4 monoclonal antibody that was previously approved by the FDA for use in patients with metastatic urothelial carcinoma who had received cisplatin-based chemotherapy. But no immune checkpoint inhibitor had demonstrated efficacy as adjuvant therapy until nivolumab.
“This trial showed a significant and clinically meaningful benefit of adjuvant systemic immunotherapy,” says Dr. Galsky. “And based on those findings, we believe nivolumab has the potential to become a new standard of care for the many patients whose urothelial cancer has invaded the muscle layer of the bladder wall.”
Featured

Matthew Galsky, MD
Associate Director for Translational Research