Tumors are comprised of a complex milieu of different cells that interact with each other in a dynamic and spatially organized manner that defines the state of the tissue. In order to thrive, malignant cells adjust the composition of their local environment. One way is by recruiting or restricting the types of immune cells that enter the tumor microenvironment (TME). A critical factor that influences tumor response to immunotherapy is the composition of the TME. Yet the different genes and pathways used by cancer cells to shape the composition and arrangement of the TME to their advantage are not fully known, and this limits the research community’s ability to target the TME as a therapeutic modality.
Identifying the genes that control the TME is a challenge because cancer cells can have thousands of dysregulated genes. Finding which ones are responsible is not an easy task. Functional genomics has helped to provide a scaled means of rapidly assessing the functions of many genes at once, but these approaches are primarily suited toward identifying genes that control particular cell-intrinsic processes. Studying more complex phenotypes at scale, especially involving how the tumor constructs its microenvironment, has not been very feasible.
This is a challenge Brian Brown, PhD, and his lab are addressing, and one for which they recently received funding through the prestigious National Institutes of Health (NIH) Director’s Transformative Research Award (TRA). Dr. Brown is Professor of Genetics and Genomic Sciences, and Associate Director of the Precision Immunology Institute, Icahn School of Medicine at Mount Sinai. The TRA is part of NIH’s High-Risk, High-Reward Research program and one of the most competitive NIH awards.
To carry out their studies, Dr. Brown and his team have created a first-of-its-kind functional platform—dubbed Perturb-map—that enables them to use high-dimensional imaging to detect many different CRISPR gene perturbations in situ. They can examine how different genes control the spatial architecture of a tumor at a cellular and tissue-level resolution. By enabling scaled multiplex analysis, Perturb-map will greatly accelerate the process of discovering genes and mechanisms that control different aspects of tumor biology.
The Brown lab intends to apply Perturb-map to one of the most pressing questions in cancer immunotherapy—namely, how tumors prevent immune infiltration and subvert immunity. Ideally, Dr. Brown’s technology will help scientists discover drugs that enable them to shrink and eliminate cancerous tumors, and help physicians determine which patients might not make good candidates for immunotherapy.
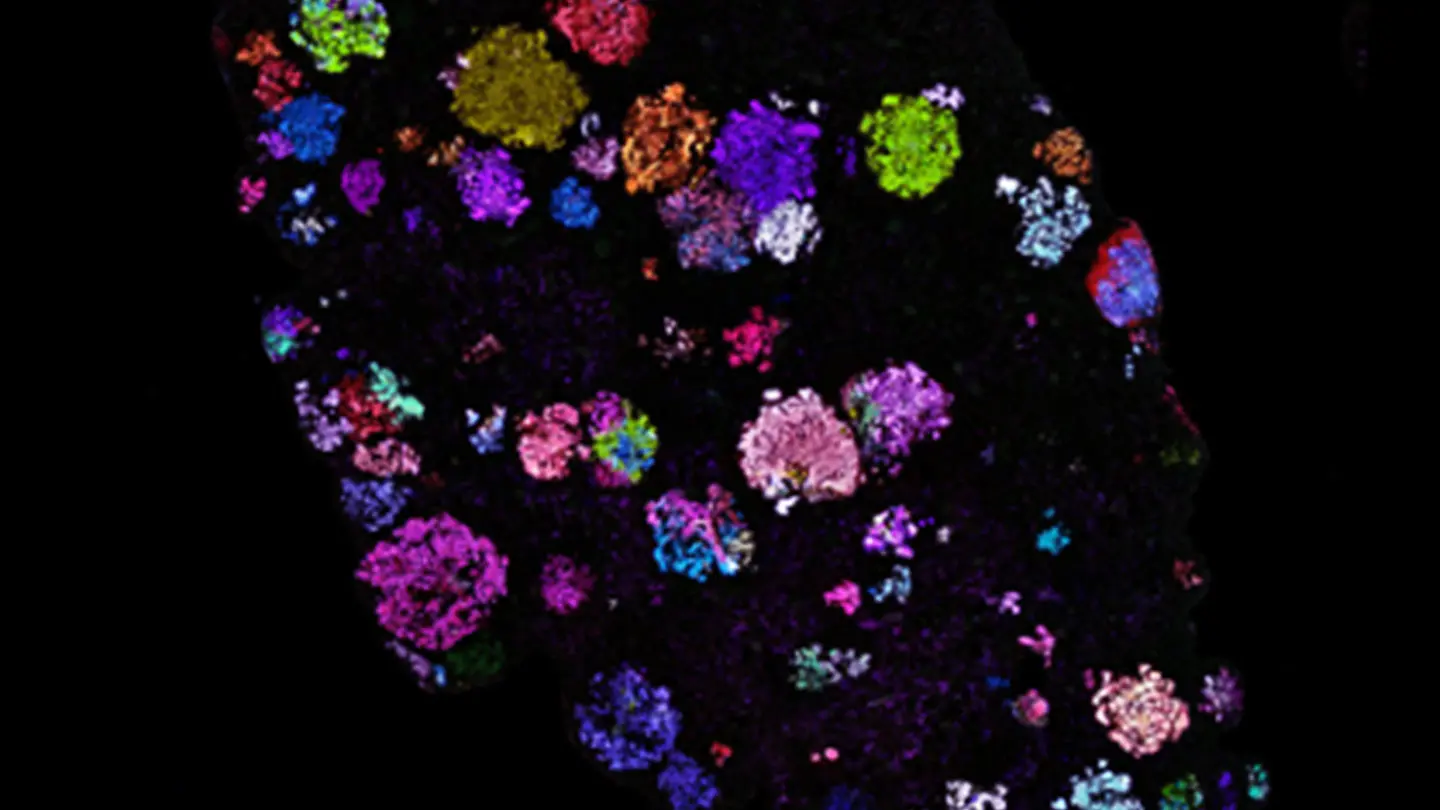
Perturb-map technology is an outgrowth of Dr. Brown’s prior work, which established a synthetic protein-level barcoding technology called Pro-Codes, which was first described in a study in the November 2018 issue of Cell. The Pro-Codes enabled CRISPR screens to be resolved with high-dimensional resolution using single-cell detection technologies such as flow cytometry and CyTOF mass cytometry. With the Perturb-map approach, they now add spatial resolution to CRISPR screens through the use of multiplex imaging methods to detect Pro-Code-expressing cells.
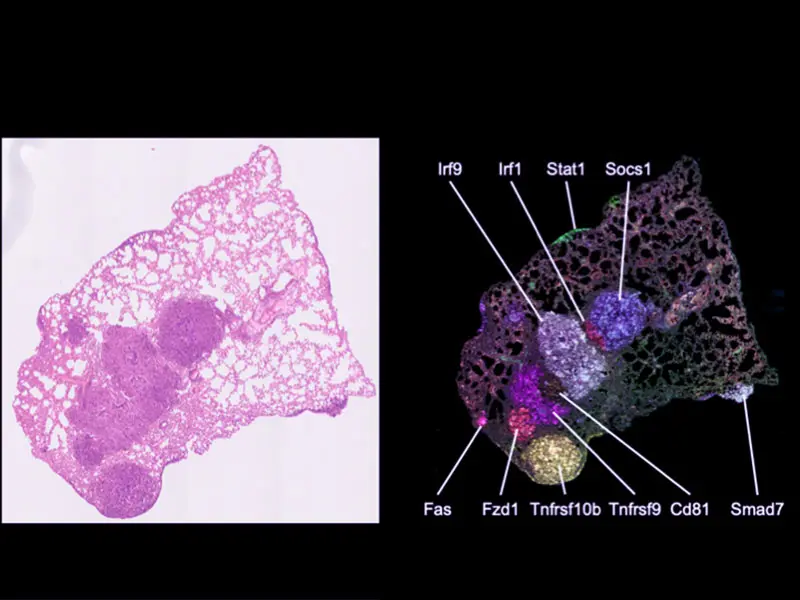
Spatial mapping of Pro-Code/CRISPR tumor lesions.
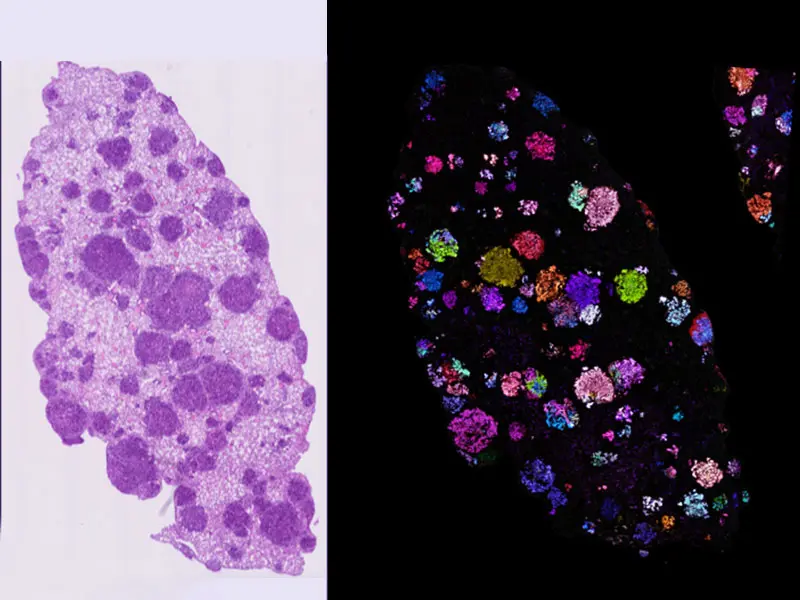
Pro-Code spatial mapping of KP lung tumors indicates lesions are highly clonal.
Featured

Brian Brown, PhD
Professor of Genetics and Genomic Sciences, and Associate Director of the Precision Immunology Institute