For millions of Americans, neuropathic pain, which can present as burning, pressing, tingling, or freezing sensations, exact a physical and mental toll that often interferes with their ability to work and engage in other routine activities. Worse yet, commonly prescribed treatments—including nerve blocks, tricyclic antidepressants, anticonvulsants, and opioids—have limited efficacy and burdensome side effects, underscoring the urgent need for innovative new therapeutic modalities.
Colorado-based medical device company Sana Health has developed a device that uses audio visual stimulation (AVS) for neuropathic pain and other neurologic and psychiatric conditions. AVS works by stimulating the central nervous system, through exposing the patient to patterns of light and sound. This process induces mental states comparable to meditation, deep relaxation, and rapid sleep onset. Studies have shown that treatment with AVS may lead to long-term reorganization of dysfunctional pain processing pathways by affecting the neural circuits responsible for the perception of pain.
The Sana Health device works on the principle of audio-visual stimulation for neuropathic pain, by sending pulses of light and sound that are specifically engineered to produce very particular frequency patterns in the brain. These stimuli are thought to have therapeutic benefits for neuropathic pain and other chronic conditions.
Mount Sinai was brought on as a lead investigator in a pivotal clinical trial for the device’s U.S. Food and Drug Administration (FDA) clearance in neuropathic pain. The AVS device is placed over the eyes during sleep, and findings from the study point toward patient benefit.
“Based on our results, we believe AVS technology may offer clear benefits in reducing neuropathic pain, especially for patients for whom conventional therapies aren’t as effective,” says Laura Tabacof, MD, Assistant Professor of Rehabilitation and Human Performance at the Icahn School of Medicine at Mount Sinai, and lead author of the study, which is currently in preprint. The device appears to offer favorable side effects and patient compliance, especially over some commonly used medicines such as opiates, which at higher doses can be very dangerous and lead to death, she adds.
Promising Results
The double-blind, randomized trial involves 64 participants, comparing changes in pain symptoms of those who received treatment from the device against those who received a sham device. Participants were assessed on pain measures at various intervals, spanning a total of 14 weeks, and were also tracked on other measures including sleep quality, depression, and quality of life.
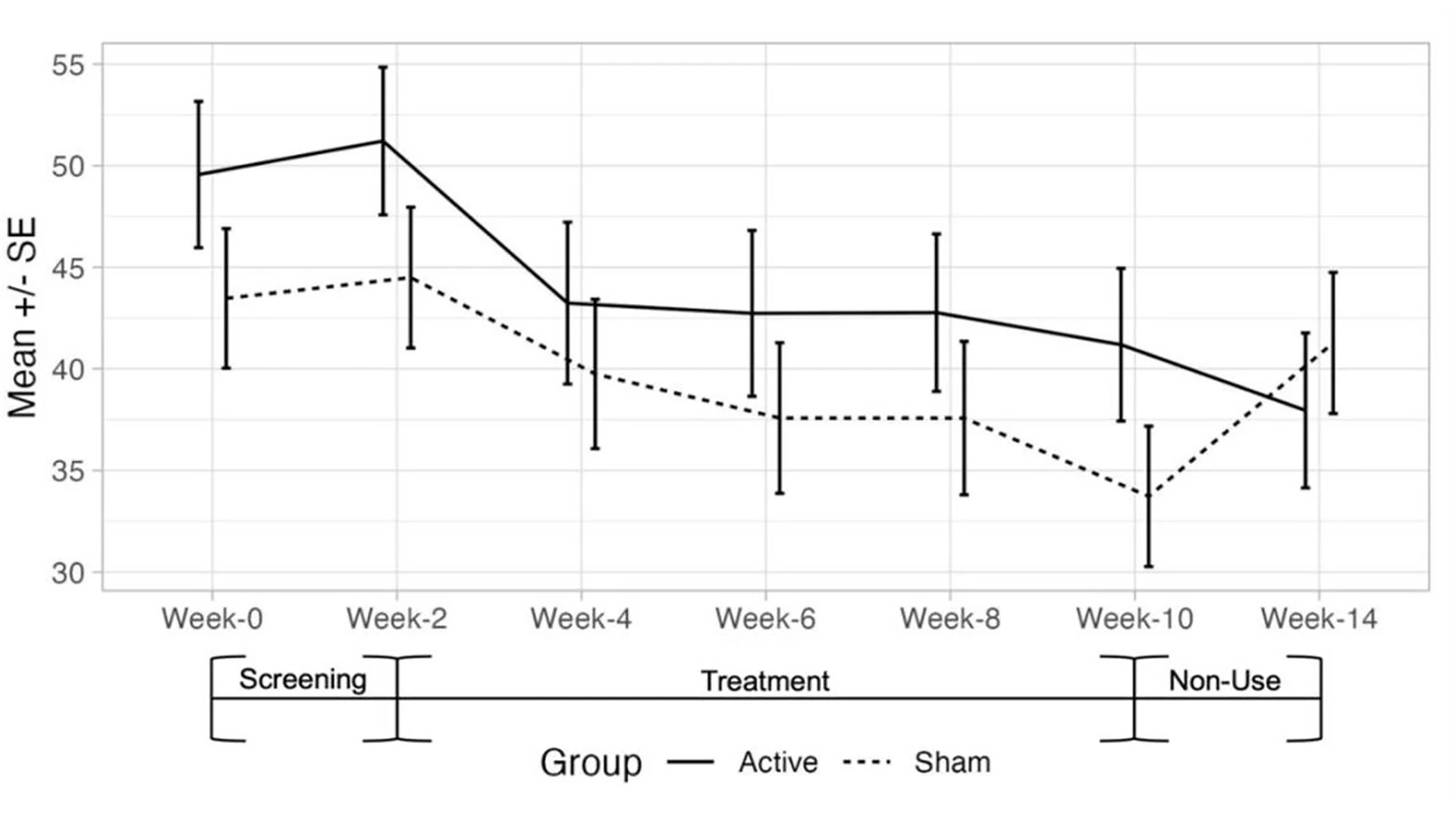
Both groups—those treated with the Sana Health device and those who received a sham treatment—showed statistically significant pain score improvements at 10 weeks, according to unpublished data. However, only the treated group saw sustained benefit from baseline, whereas the sham group reported pain scores that retreated toward baseline.
Both the group with the actual AVS device and the one with the sham device showed significant improvement (p ≤ .001) in pain scores at week 10, compared to baseline at week 2. In a cross-comparison, the treated group results were not significantly different from the sham group. However, after patients were not given any treatment and reassessed at the 14-week mark, only the treated group showed sustained pain score improvements, whereas the sham group scores returned to baseline—a cross-comparison that was significant (p = .01).
The data—currently unpublished—signal a promising approach to pain management; but also shed light on neuropathic pain.
“Our results support the theory of a central mechanism behind neuropathic pain, with the AVS device potentially reversing some of the maladaptive neuroplasticity associated with neuropathic pain,” says Dr. Tabacof.
Neuropathic pain is a lesion or disease of the body’s somatosensory system, believed to affect up to 10 percent of the general population. Burning and pressing pain are two of the most common symptoms, present in up to 70 percent and 63 percent, respectively, of patients with neuropathic pain across a wide range of pathologies. Pharmacological treatment remains the first-line option for both peripheral and central neuropathic pain, though clinical effectiveness of medications is only moderate.
The collaboration between Mount Sinai and Sana Health came to be when David Putrino, PhD, Director of Rehabilitation Innovation at Mount Sinai, was intrigued by early anecdotal data from the device’s prototype. Dr. Putrino and Sana Health jointly designed the protocols for the clinical trial that would be the device’s basis for FDA clearance in neuropathic pain. In addition, Mount Sinai is now running a clinical trial using the same AVS technology to study a cohort of 50 people with chronic pain who have Lyme disease.
“It is promising that a new device, with a good safety profile, can potentially address neuropathic pain in a noninvasive way by targeting a key mechanism that drives the condition,” says Dr. Tabacof. “It’s good news for a population that is in desperate need of new treatments.”
Featured

Laura Tabacof, MD
Assistant Professor of Rehabilitation Medicine and Human Performance