Long COVID, defined as a chronic disease state that develops after a COVID-19 infection and lasts for at least three months, affects an estimated 65 million people worldwide. With often-debilitating effects, affecting a broad range of organs, scientists are delving deeper into the pathobiology of the disease as a critical step toward developing new therapies.
Two new groundbreaking studies from Mount Sinai are offering hope to patients by revealing windows into the biological sex-based characteristics of Long COVID and the role of autoantibodies (AABs) as a key driver of a wide range of neurocognitive and neurological pathologies associated with the disease.
Symptom Burden Differences Among Sexes
The first of the studies, published in medRxiv, has identified the contribution of biological sex to the prevalence and symptom manifestation of Long COVID.
More than four years after the outbreak of the COVID-19 pandemic, research has established that females are significantly more likely than males to present with Long COVID. What’s unknown is whether immunological traits underlying long COVID vary between sexes and whether those traits can explain differences in disease symptomology of males and females.

Long COVID, despite affecting many people following the pandemic, is still woefully understudied. David Putrino, PhD, and his team are uncovering mechanisms of the chronic disease state and are in the process of publishing two studies that could make headway toward providing patients effective relief.
Drawing on survey data and immunoendocrine profiles of 185 males and females with and without Long COVID, researchers from Mount Sinai and Yale School of Medicine found not only different sets of symptoms between the sexes, but distinct patterns of organ system involvement, with females suffering a higher symptom burden.
Machine learning approaches helped researchers determine that women with Long COVID had increased frequencies of exhausted T cells, cytokine-secreting T cells, higher antibody reactivity to latent herpes viruses, and, perhaps most significantly, lower testosterone levels than their control female counterparts.
“Since the pandemic, there hasn’t been much investigation into hormonal differences between men and women with infection-associated chronic conditions, or the role of testosterone in driving symptoms such as chronic fatigue syndrome, headaches, and neurological conditions,” says David Putrino, PhD, Professor of Rehabilitation and Human Performance at the Icahn School of Medicine at Mount Sinai, and co-author of the study.
“Our work suggests that testosterone levels, especially in females, can lead to profound changes in overall Long COVID symptom burden, and that females and males with Long COVID experience a relative decrease in their nondominant sex hormones," he says. “This means that females with the most severe symptoms are likely to have extremely low testosterone.”
Underscoring this phenotype was the discovery by researchers that lower testosterone levels in females with Long COVID were associated with higher antibody reactivity to the latent herpes viruses EBV, HSV-2, and CMV, while in males, lower estradiol (which is often a proxy for relative testosterone levels in men) was associated with higher reactivity to EBV and HHV6-b.
These findings suggest that testosterone and estradiol levels in people with Long COVID may play a crucial role in managing immune responses to latent viruses. A hormonal-immune imbalance that is triggered following a SARS-CoV-2 infection may also have a link to predisposing people with Long COVID to autoimmune-related conditions.
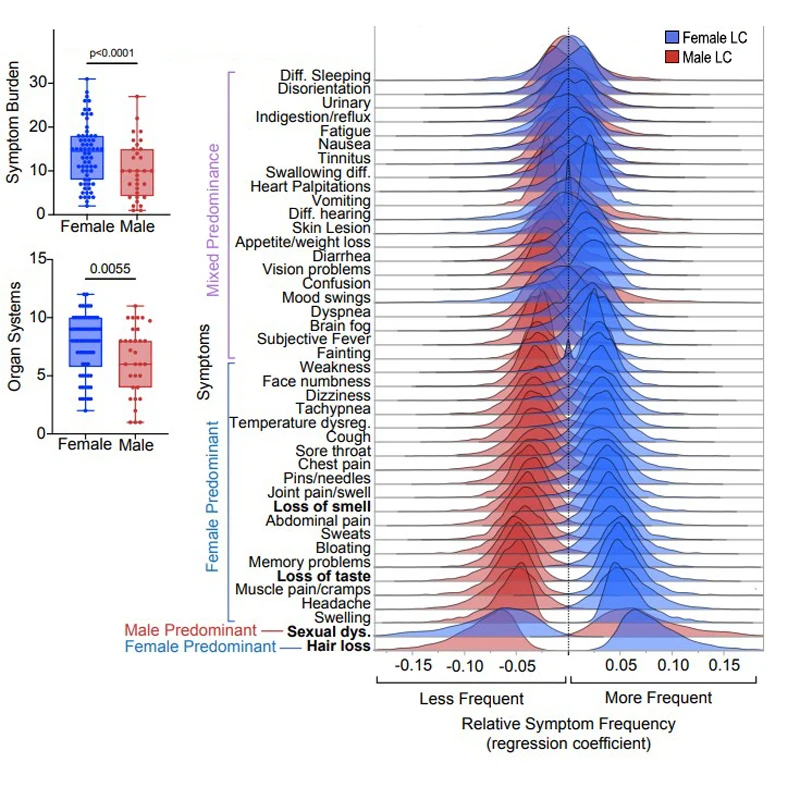
Females with Long COVID showed significantly higher symptom burden and organ system involvement compared to males. The top distinguishing symptoms of Long COVID by sex were hair loss in females and sexual dysfunction in males.
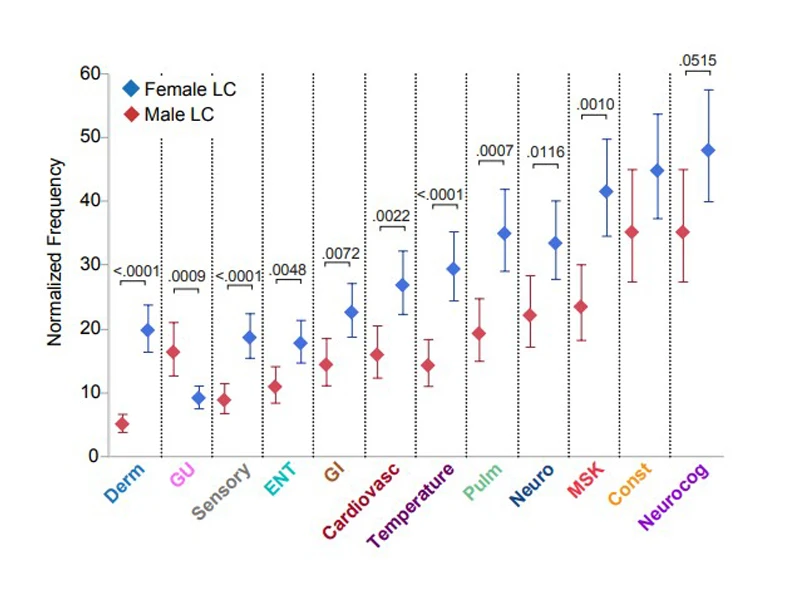
Neurological and neurocognitive symptoms were reported with similar frequency in both females and males with Long COVID, but temperature-related symptoms and musculoskeletal symptoms were more likely to be reported by females. By contrast, ear nose and throat symptoms were most reported by males.
Building on these findings could point to a potential pathway to mitigate Long Covid symptoms through hormonal replacement therapy (HRT). However, because HRT is so complex, it’s not suitable for everybody and needs to be personalized, cautions Dr. Putrino, who is also the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness.
“For instance, one person with Long COVID may need to be prescribed progesterone alongside their testosterone, while another will require testosterone on its own,” says Dr. Putrino. “Our study opens the door to a promising clinical trial that targets looking into hormone replacement therapy for people living with Long COVID.”
Impact of Autoantibodies on Long COVID
Other potential treatment approaches for Long COVID have emerged in a second Mount Sinai-led study, published in medRxiv, examining the link between AABs and neurological symptoms from Long COVID. AABs are antibodies produced by the immune system that are directed against the individual's own proteins, and many autoimmune diseases are associated with AABs.
Diverse and functional AABs are often found during the acute phase of a SARS-CoV-2 infection due to bystander activation or molecular mimicry. Normally, these levels subside as the immune system stabilizes. However, a number of large studies have reported a 20 percent to 40 percent increased risk of multiple new-onset autoimmune diseases after COVID-19, leaving scientists to wonder if persisting AABs may trigger symptoms in some patients with Long COVID, even if these individuals don’t meet the classic clinical diagnosis of autoimmune disease.
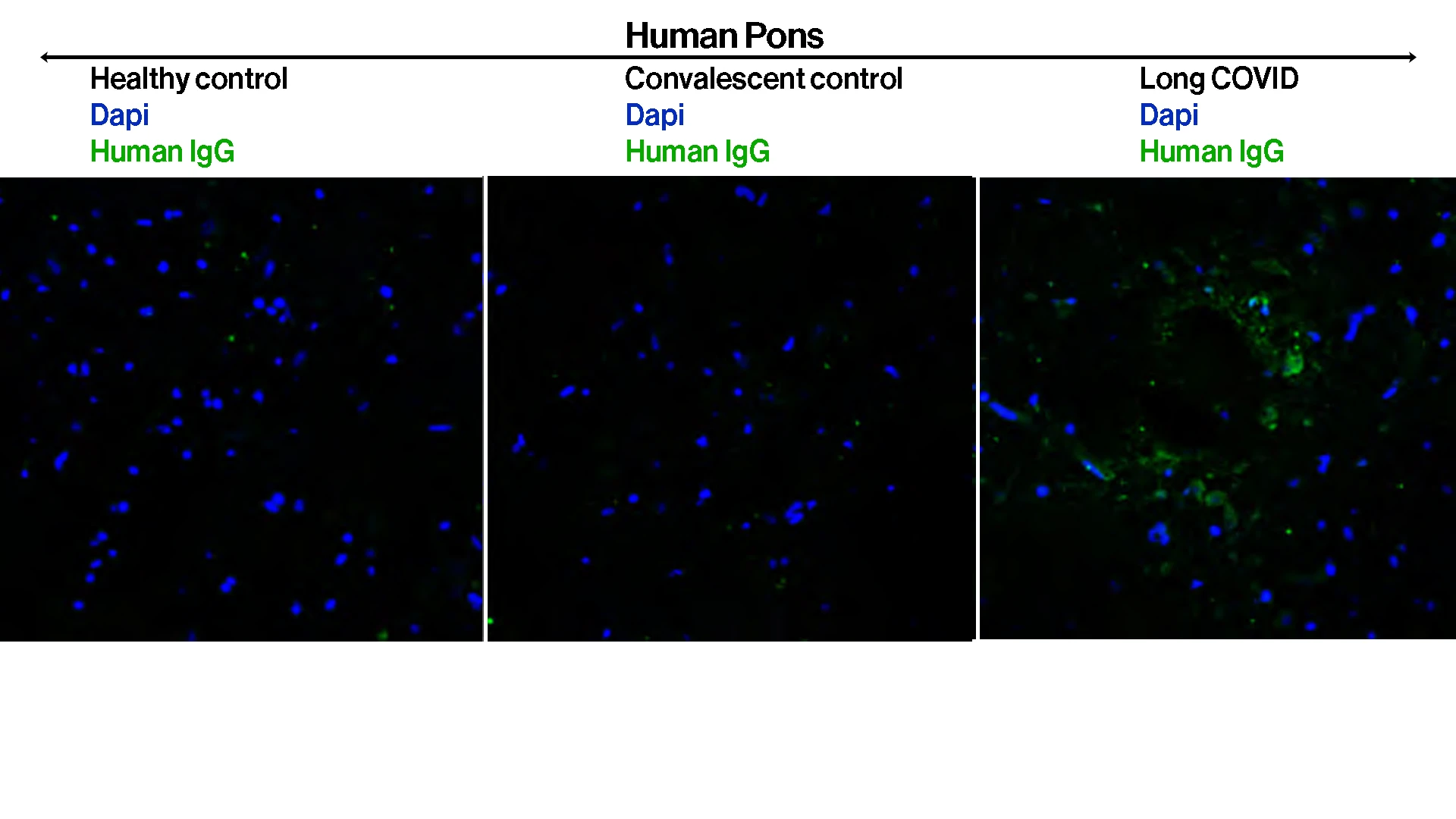
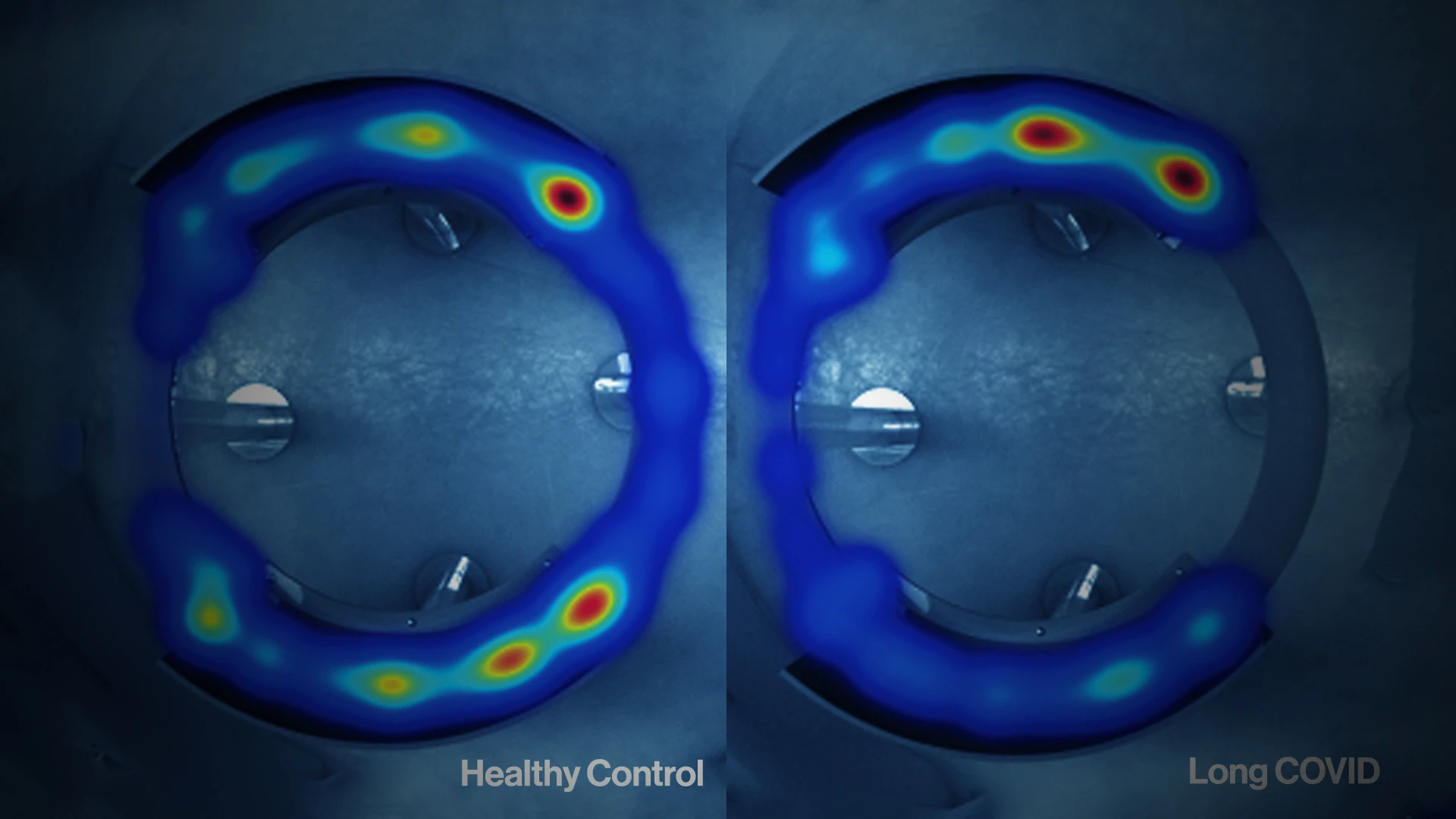
To evaluate if study participants had functional autoantibody profiles that could provoke their
Long COVID-associated neurological symptoms, researchers purified IgG from plasma and performed immunofluorescence analyses using healthy human tissues. Participants' IgG showed reactivity against human pons—seen as green fluorescence in the slides—as well as in various mice tissue as part of protocol.
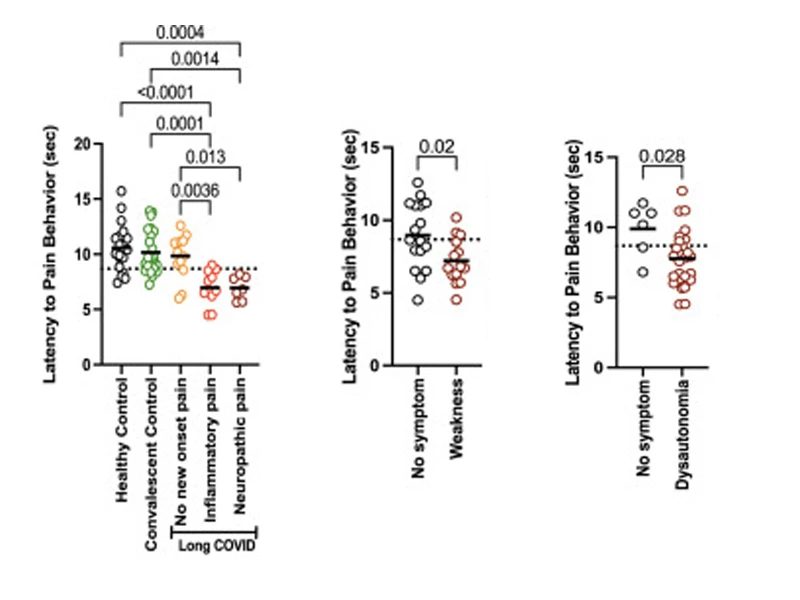
Mice receiving IgG from those diagnosed with chronic pain displayed enhanced pain sensitivity at the hot plate test, with 85% of the mice with the pain phenotype having received IgG from participants with Long COVID who self-reported new-onset pain since their Long COVID diagnosis (left). Additionally, 55% of the mice who received IgG from patients who reported weakness, and 90% of mice who received IgG from patients who reported dysautonomia scored positive for pain (middle and right).
Collaborating with Yale School of Medicine, Mount Sinai researchers evaluated the presence of AABs and their pathological potential in Long COVID. Through use of a comprehensive human autoantigen microarray, the team’s data showed the pivotal role of AABs as a key driver of neurological disorders in Long COVID, suggesting their utility as potential biomarkers in diagnosing this Long COVID endotype.
To arrive at those findings, scientists transferred purified immunoglobulin G (IgG), the most common type of antibody in human blood, from people with Long COVID and healthy controls into mice, and recapitulated key neurological symptoms described by patients with long COVID.
“We learned that mice receiving IgG from people with Long COVID were much more likely than our control groups to develop balance issues, loss of grip strength, increased pain sensitivity, and weakness in the limbs,” notes Dr. Putrino, co-senior author of the study.
Another interesting finding was that researchers saw different neurological symptoms emerging in mice depending on the autoantibody and self-reported symptom profile of the human contributors.
A key question that remains for scientists, though: is it just an immune system that has been knocked out of balance, triggering this prolonged autoantibody response, or is it a systemic pathogen, perhaps hidden in tissue, that causes prolonged proliferation of AABs?
The researchers continue to explore how they might be able to use intravenous immunoglobulin (IVIg) as a potential treatment by neutralizing and clearing AABs. Benefits from IVIg treatment have been reported in Long COVID patients with small fiber neuropathy, but responses to IVIg are highly variable and can come with drawbacks, including access and cost issues.
This is why the Mount Sinai and Yale teams have expressed great interest in a new class of drugs known as neonatal FC receptor (FcRn) inhibitors with the potential to degrade circulating AABs without suppressing the immune system or IgG.
“AABs are not widely recognized as a driver of Long COVID neurological symptoms,” says Dr. Putrino. “But our data point to key pathological roles of AABs in driving neurological phenotypes of Long COVID in vivo, and underscore the urgent need for further studies that can move the needle toward finding effective treatments for a disease that has upended the lives of millions of people around the world.”
Featured

David Putrino, PhD
Director of Rehabilitation Innovation, and Professor of Rehabilitation and Human Performance