Despite the severity and complexity of neuropsychiatric illnesses, such as schizophrenia, treatment methods have remained largely the same for decades. “It’s embarrassing that we are still treating schizophrenia with the same medications that were discovered in the 1950s and 1960s. We desperately need new directions for developing treatments for individuals suffering from schizophrenia and other serious mental illnesses,” says Panos Roussos, MD, PhD, Professor of Psychiatry, and Genetics and Genomic Sciences, at the Icahn School of Medicine at Mount Sinai, and Director of the Center for Disease Neurogenomics.
Under the auspices of the PsychENCODE Consortium, Dr. Roussos and his colleagues are advancing into phase two of that goal, taking advantage of new tools and methods that enable a deeper dive into the molecular biology of neuropsychiatric diseases. The National Institute of Mental Health (NIMH) launched PsychENCODE in 2015 to advance research exploring the molecular underpinnings of neuropsychiatric diseases. Findings from the first phase of the project, published in a special issue of Science in 2018, were largely based on molecular data that had been previously collected in previous work.

The special May edition of Science included nine of 14 papers in phase two of PsychENCODE's studies.
For phase two, Mount Sinai researchers were involved in two projects, which were reported in a special edition of Science dedicated to PsychENCODE in May.
Single-Cell Analysis of Schizophrenia Brains
In one of the new studies, Dr. Roussos, John Fullard, PhD, Assistant Professor of Psychiatry, and Genetics and Genomic Sciences, at Icahn Mount Sinai, and their colleagues compared molecular changes in brain tissue samples from donors with schizophrenia and healthy controls. In the past, such analyses involved examining all the different cell types from each sample together. Though it was an important start, the combined approach limited the usefulness of the findings. “The brain contains many different cell types, and we wouldn’t expect a disease to affect each of those cells in the same way. By looking at everything mixed together, we were missing things that might affect rarer, but no less important, cell types,” Dr. Roussos explains.
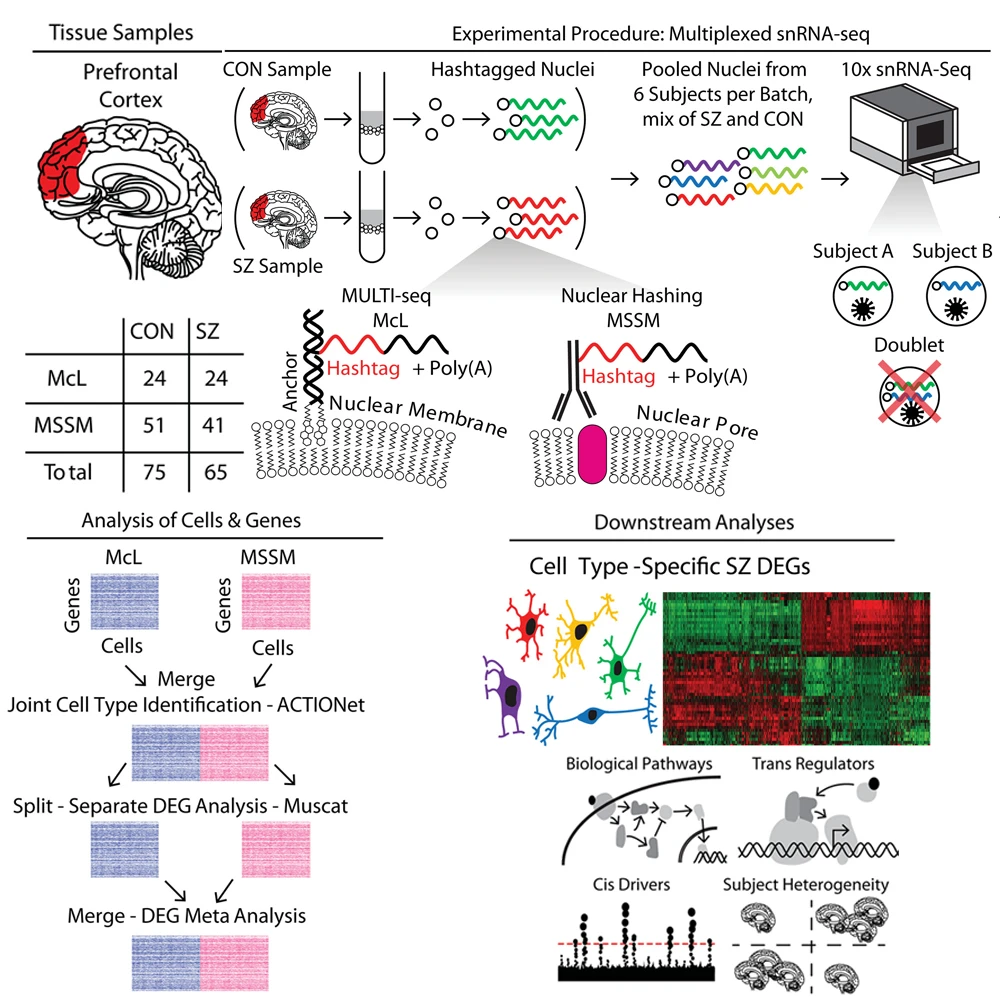
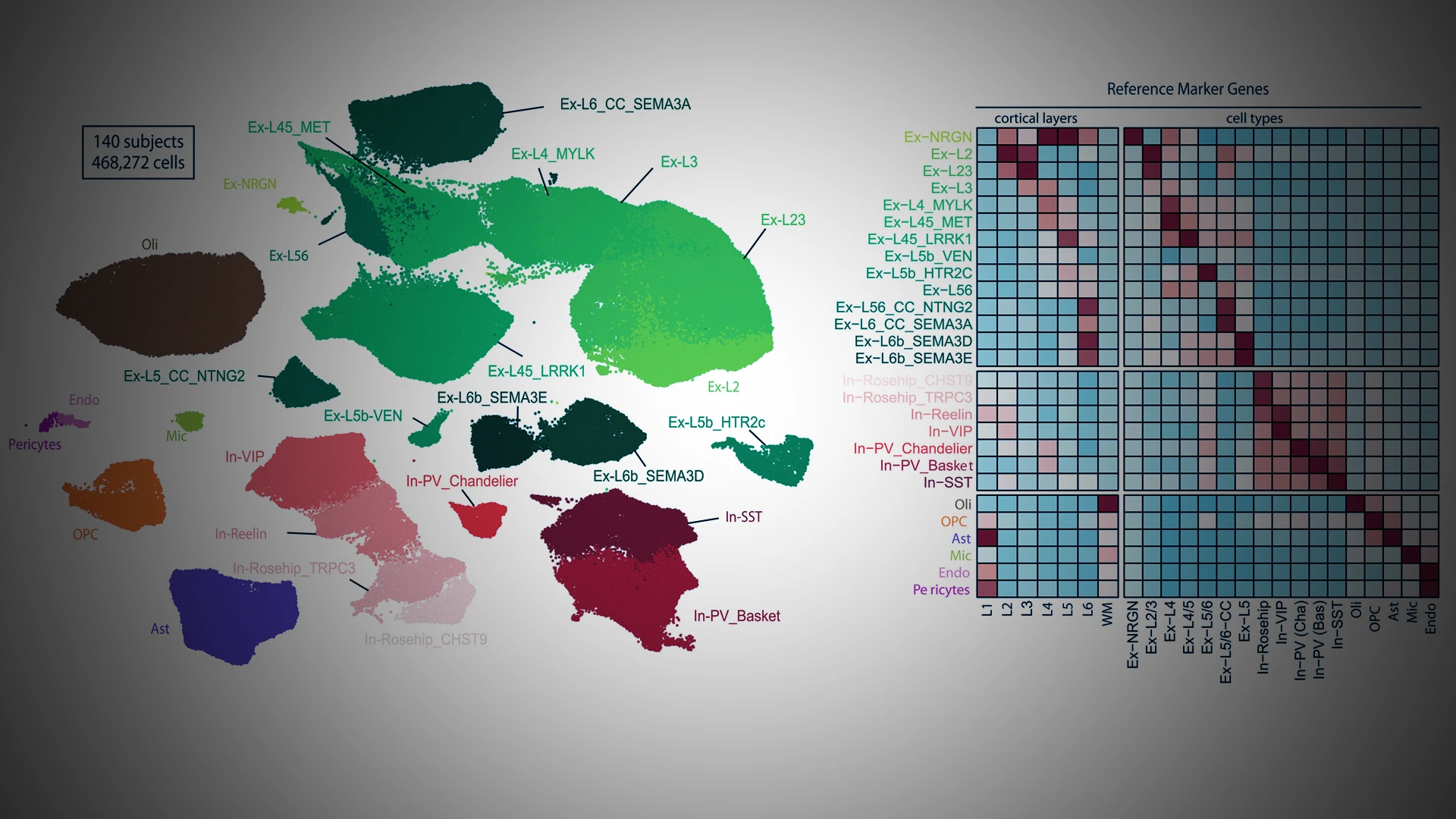
The study involved single-nucleus RNA sequencing of postmortem prefrontal cortex tissue samples from the Icahn School of Medicine at Mount Sinai and McLean Hospital in Massachusetts. The study pooled cases and controls to increase number of cells captured, and performed downstream analyses of biological pathways, cis- and trans-regulatory factors related to cell type–specific schizophrenia differentially expressed genes.
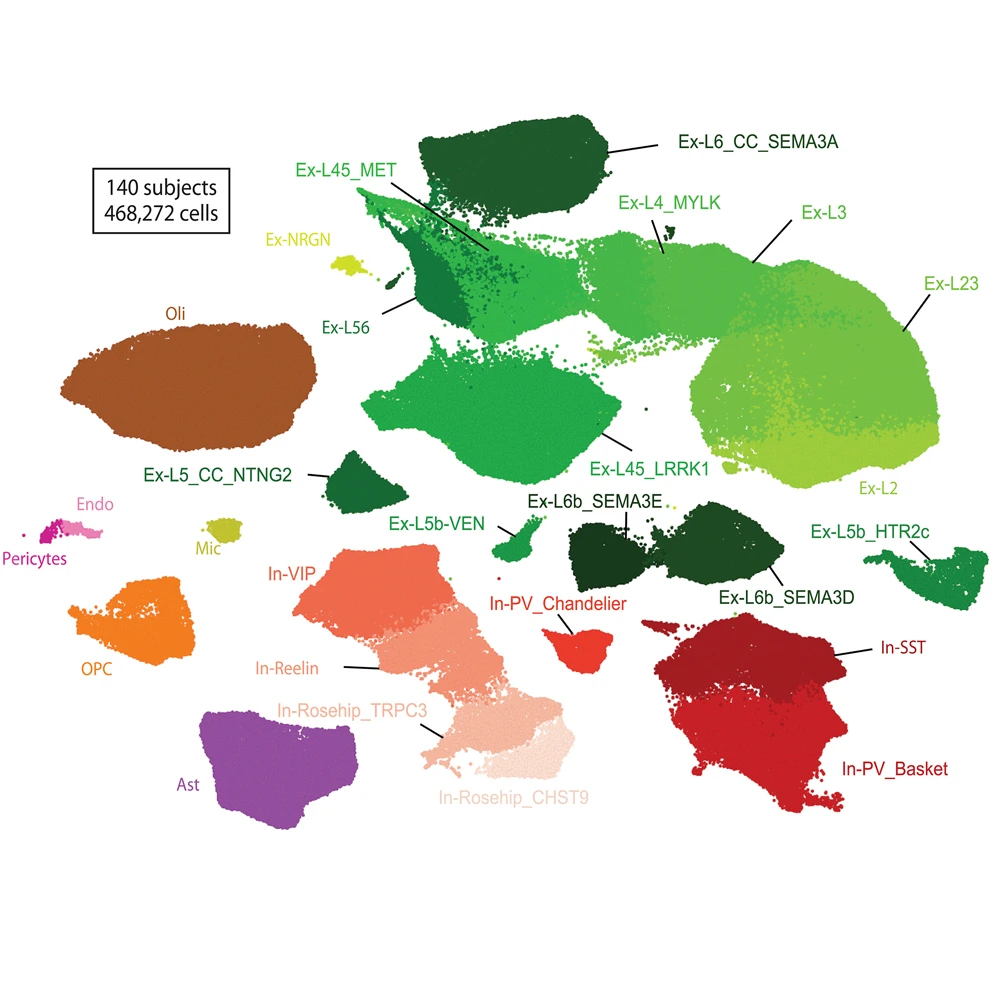
From 140 subjects, the team achieved 468,727 transcriptomes, including 206,014 nuclei from individuals with schizophrenia and 262,713 from healthy individuals. In this plot of putative cell types, green and red clusters represent excitatory and inhibitory subtypes of neurons, respectively, with darker shades indicating an association with deeper cortical layers.
The new analysis, co-led in collaboration with partners at The Eli and Edythe L. Broad Institute of MIT and Harvard, took advantage of single-cell approaches to analyze highly complex tissues, such as the human brain. This allowed for identification of transcriptional alterations associated with each unique cell type. “This is by far the largest single-cell analysis of schizophrenia to date,” Dr. Roussos says. “And by partnering with MIT, we were able to compare independent findings from two different patient cohorts to verify that they were consistent from one dataset to another.”
The result was a more comprehensive, detailed understanding of the molecular alterations associated with schizophrenia. “We observed significant molecular changes within a specific type of neurons, excitatory neurons, from the brains of people with schizophrenia. After identifying those molecular changes, we could look one level up to identify the transcription factors that drive many of those changes,” Dr. Fullard says. “This has allowed us to identify important upstream regulators that could serve as targets for drug development.”
Regulatory Mechanisms Underlying Brain Diseases
In a second study, Dr. Roussos and Mount Sinai colleagues, including Biao Zeng, PhD, Instructor of Psychiatry, and Genetics and Genomic Sciences, and Gabriel Hoffman, PhD, Associate Professor of Psychiatry, and Genetics and Genomic Sciences, analyzed samples from the brains of individuals with and without neuropsychiatric diseases to generate the largest epigenetic analysis of the human brain. The result is a population-scale map of the regulatory components of the brain that captures insights into the etiology of brain disease.

Chromatin accessibility was measured in 1,932 aliquots of sorted neurons and non-neurons from 616 individuals across four distinct brain regions: prefrontal cortex, anterior cingulate cortex, superior temporal gyrus, and parahippocampal gyrus. The discovery of cell type–specific chromatin accessibility quantitative trait loci helps elucidate the molecular mechanisms that drive disease risk.
“We can now begin to understand how genetic variation among individuals might affect epigenetic regulation,” Dr. Roussos says. “This work provides a reference map for exploring the molecular mechanisms that drive the development of serious mental illness.”
One key finding was the discovery that genetic variation plays a very different role in epigenetic regulation of chromatin accessibility in neurons versus non-neurons. “There are dramatic cell-type differences in the ways that genotype affects those molecular markers,” Dr. Zeng says. “This finding underscores the need for more large-scale single-cell analysis of the human brain in future work.”
An important aspect of the PsychENCODE project’s second phase has been the use of new tools to validate findings. In this study, the researchers artificially overexpressed the known genetic variants in neurons from stem cells. Manipulating gene expression allowed them to assess whether those variants affect functional outcomes. “With this kind of closed-circuit approach, we can discover, validate, and then nominate new targets that we can now follow up on with more mechanistic studies,” Dr. Hoffman says.
The epigenome is an attractive target for drug development, Dr. Roussos adds. Changing the expression of an epigenetic modifier requires a smaller, easier-to-deliver molecule than is needed for traditional gene therapy. And because epigenetic regulomes are very specific to different cell types, targeting those regulomes in the brain may reduce unwanted effects on other cell types elsewhere in the body.
Researchers are already following this new regulome roadmap into uncharted territory, with the goal of uncovering potential targets for new and better therapies. “Now, for the first time, we can link the disease and genetic architecture with different cell types and epigenetic regions,” Dr. Roussos says. “It’s the first step we needed in order to translate our findings into new treatments.”
While all the PsychENCODE Consortium partners are making important contributions to the research program, Mount Sinai is especially well suited to the effort, Dr. Roussos adds. “Advancing the study of complicated neuropsychiatric diseases requires expertise in psychiatry, neuroscience, and genomics,” he says. “Mount Sinai has a wealth of amazing expertise in all of those areas, and it’s the kind of place that brings scientists together to make these very complex studies happen.”
Featured

Panos Roussos, MD, PhD, MS
Professor of Psychiatry, and Genetics and Genomic Sciences

John Fullard, PhD
Assistant Professor of Psychiatry, and Genetics and Genomic Sciences

Gabriel Hoffman, PhD
Associate Professor of Psychiatry, and Genetics and Genomic Sciences

Biao Zeng, PhD
Instructor of Computational Biology