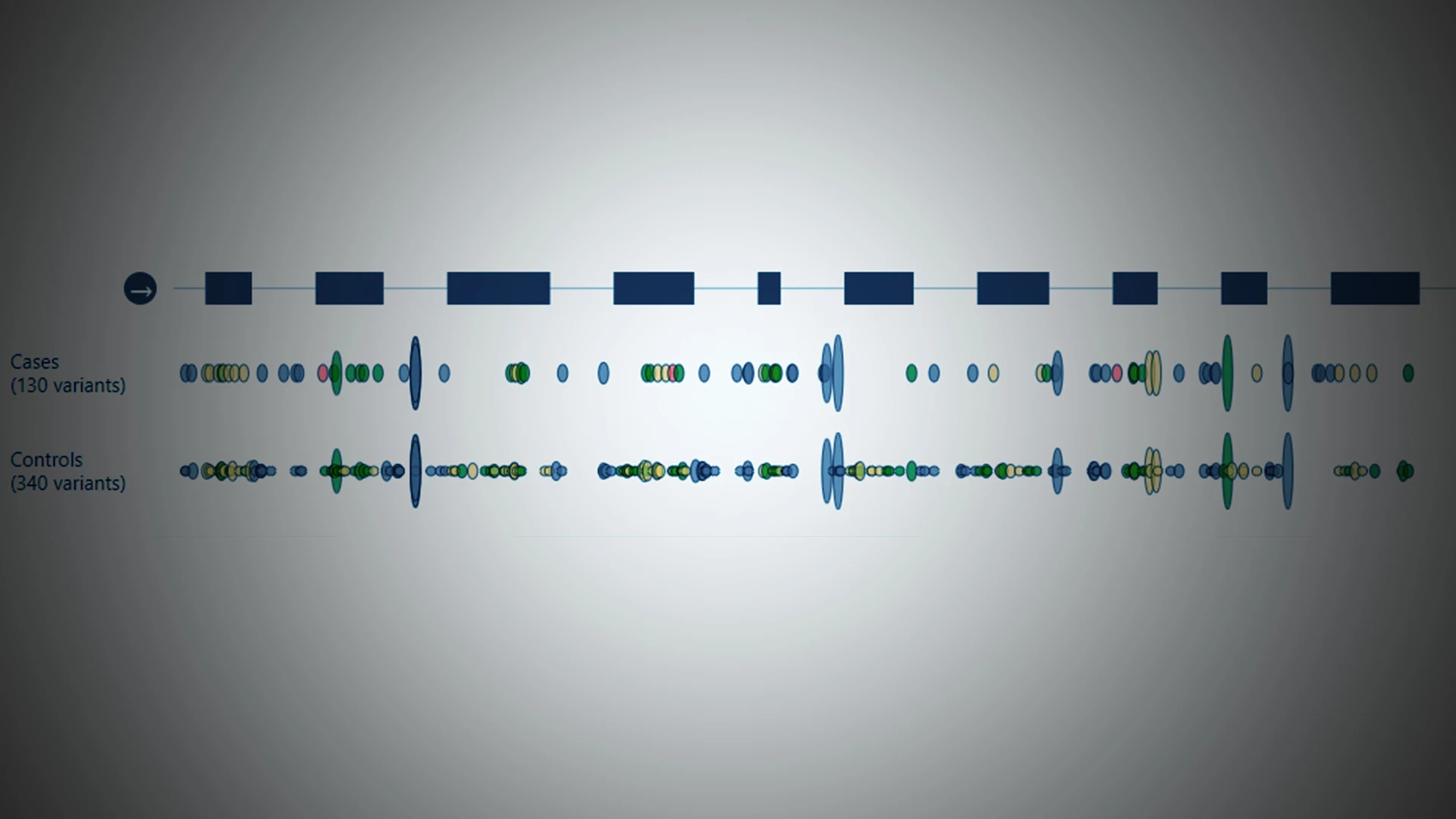
Looking through Mount Sinai’s BioMe biobank for patients with 10 rare genetic mutations implicated in psychosis and schizophrenia, Alexander Charney, MD, PhD, found 61 individuals. Intriguingly, he noted that approximately five of them had a defective variant of GRIA3, a glutamate receptor that, when coded improperly, has known links to schizophrenia.
“We were not expecting to find that,” says Dr. Charney, Associate Professor of Psychiatry, Genetics and Genomic Sciences, Neuroscience, and Neurosurgery at Icahn School of Medicine at Mount Sinai. The Schizophrenia Exome Sequencing Meta-Analysis (SCHEMA) consortium, in which Mount Sinai participated, had identified the 10 rare mutations through sequencing more than 24,000 and more than 97,000 controls worldwide. Of the variants, GRIA3 was assessed to hold among the highest odds ratio for schizophrenia risk (48), and among the lowest allele frequency in the general population (approximately 1x10-5).

Alexander Charney, MD, PhD, with lab technician Vibhuti Patel.
It was a surprise that a single biobank had this many people with the GRIA3 variant, but even more so that they were all members of the same family. “This is a family that has some members who have both the variant and have psychosis or intellectual disabilities and other members who have the variant but have no signs of disease,” Dr. Charney says. “That creates an opportunity to ask powerful questions about the molecular mechanism of psychosis so we can gain insights on the risk factors for disease development and potential therapeutic interventions.”
Understanding those molecular mechanisms is a key focus of both the Jeff and Lisa Blau Adolescent Consultation Center for Resilience and Treatment and its executive director, Dr. Charney, who leads the Center in tandem with its founding director, Rene S. Kahn, MD, PhD, Esther and Joseph Klingenstein Professor and System Chair of Psychiatry.
A ‘Population-Driven’ Study
The family-based genetic study Dr. Charney is conducting is also looking at these mutations from a population perspective based on shared characteristics. The 61 patients he identified with the mutations came from genome sequencing of 50,000 patients in the biobank. The initial plan was to study the patients to see how these mutations play a role in schizophrenia, says Dr. Charney. “Based on what we found, we developed a new protocol to study each patient and their extended families to gain insights as to why some people who have this mutation develop disease and others do not.”
The goal is to enroll as many family members of the 61 patients as possible. Each participant will undergo a standard psychiatric interview to assess whether there are conditions that might disqualify them from a schizophrenia diagnosis. Participants will also undergo a blood draw and skin biopsy, and the collected cells will be reprogrammed to create human pluripotent stem cells, which have the potential to grow into any cell or tissue in the body.

Dr. Charney with program manager Nicole Simons and lab technician Vibhuti Patel.
Using a process called directed differentiation—a bioengineering method in which specific growth factors or small molecules are administered to the pluripotent stem cells—Deepak Kaji, MD, PhD, a resident and instructor in the Department of Psychiatry, will grow the stem cells into brain organoids. These organoids recapitulate the brains of study participants and will enable him to look at how genetic mutations factor into the pathophysiology of psychosis and schizophrenia.
“Essentially, this technology enables us to subject a patient’s brain to a battery of electrophysiological tests without having to open the skull,” says Dr. Kaji. “Our hypothesis is that we will see differences in the synaptic connectivity of neurons in patients with rare variants. Deleterious changes in brain connectivity tend to play a significant role in psychiatric diseases such as schizophrenia. If we see that a certain mutation changes the way neurons conduct ion currents, we could consider therapeutic agents that force neurons to conduct currents as they would under normal physiological conditions."
This undertaking has the potential to enable examination and characterization of the genes implicated in schizophrenia to an extent never before possible—and Mount Sinai is already developing a bigger database to advance that work. An effort is underway to enroll 1 million people to profile their genomes, vastly increasing the opportunities to study the molecular mechanisms associated with schizophrenia.
Given that Mount Sinai serves a racially and ethnically diverse population, this database will be broad enough to enable studies that address disparities in mental health research. That, Dr. Charney indicates, could have a significant translational impact.
“We have people who have made donations to Mount Sinai’s biobank to further our research, and we want to be able to offer them something in return for that, such as access to an experimental therapeutic that could result in positive outcomes for them,” Dr. Charney says. “We need to translate the knowledge we are gaining on molecular mechanics into new treatments.”
Featured

Alexander Charney, MD, PhD
Associate Professor of Psychiatry, Genetics and Genomic Sciences, Neuroscience, and Neurosurgery

René S. Kahn, MD, PhD
Esther and Joseph Klingenstein Professor and Chair, Department of Psychiatry and Behavioral Health