While CAR-T cell therapy is effective for many patients with blood cancers, it is not yet effective at treating solid tumors, such as ovarian, pancreatic, lung, or breast cancers.
One of the biggest challenges of developing a CAR-T therapy for solid-tumor cancers is that the cancer cells are so similar to normal cells, there isn’t a unique molecule that the therapy can target to distinguish between the two, said Brian Brown, PhD, Director of the Icahn Genomics Institute and Associate Director of the Marc and Jennifer Lipschultz Precision Immunology Institute (PrIISM) at Mount Sinai.
Another challenge, he said, is that solid tumors have an “immunosuppressed” environment that prevents the immune system from attacking the cancer cells. They do so by recruiting macrophages to reside in the tumors, which are able to stop T cells from killing the cancer cells.

Miriam Merad, MD, PhD, and Brian Brown, PhD
Together with Miriam Merad, MD, PhD, Director of PrIISM and co-Director of the Cancer Immunology Program in The Tisch Cancer Institute at Mount Sinai, Dr. Brown and their team have engineered CAR-T cells to kill tumor macrophages by programming them with a CAR that recognizes a protein found on the surface of tumor macrophages. Their findings were published in Cancer Immunology Research in October 2022.
CAR-T cell therapy is a leading type of gene and cellular immunotherapy that has transformed the treatment of blood cancers, such as leukemia and lymphoma, Dr. Brown said. It works by equipping the patient’s own T cells with a chimeric antigen receptor on their surface, which makes those T cells seek out and kill cancer cells that have a target molecule on their outer surface.

The CAR-T cells engineered by Dr. Brown’s team have been observed to preferentially gather in tumors and not in healthy tissues, sparing the latter of toxicity. In the tumor, the CAR-T cells kill off the tumor’s macrophages, making the tumor more vulnerable to the patient’s own immune system and reducing tumor growth in mouse models of lung, pancreatic, and ovarian cancers. But the team found this effect dependent on the CAR-T cells producing the inflammatory cytokine interferon gamma locally.
“The key to our strategy is the localization of the CAR-T cells,” said Dr. Brown. When CAR-T cells get concentrated in the tumor, they make the tumor even more conducive to attack and elimination by the recipient’s own T cells.
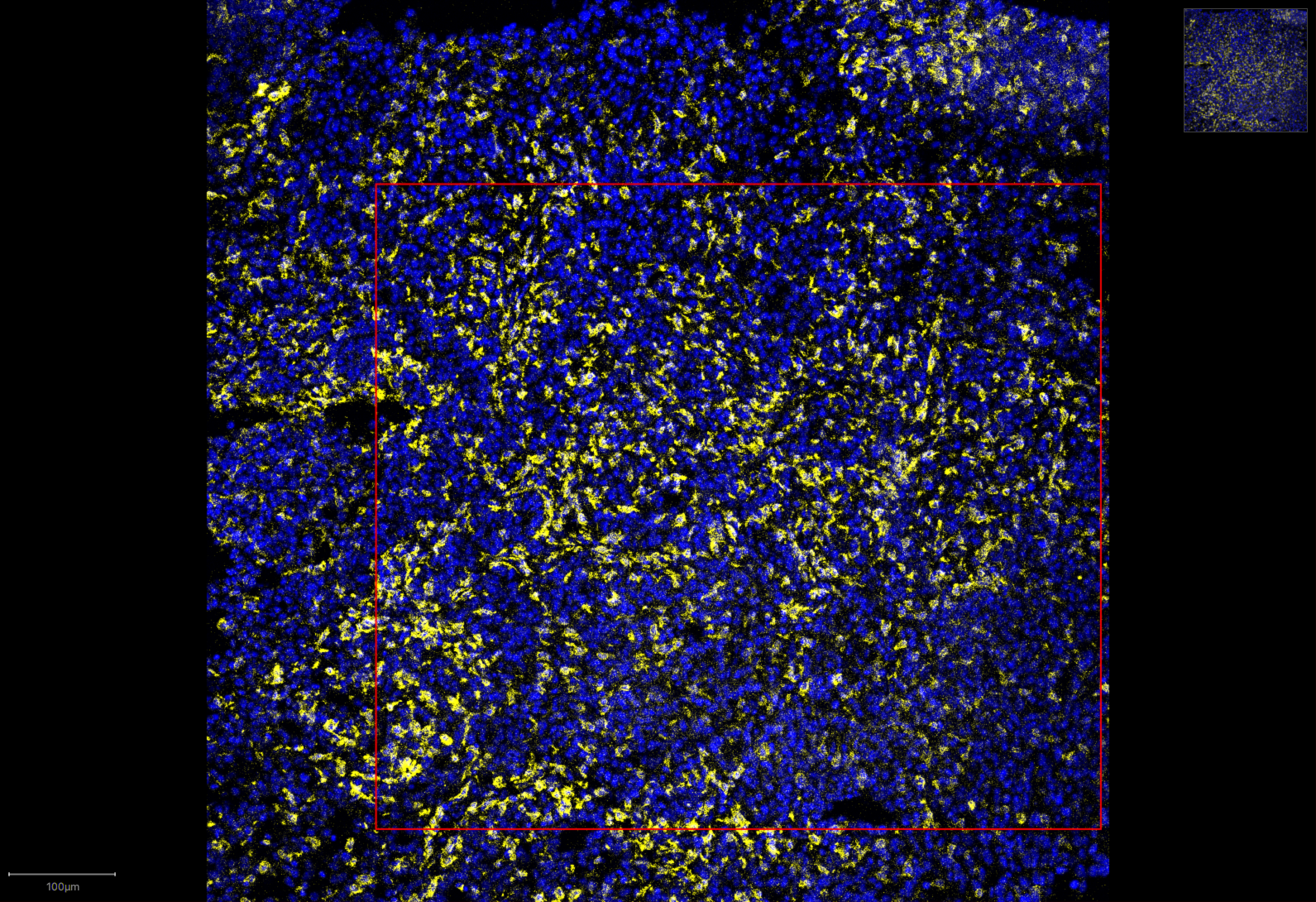
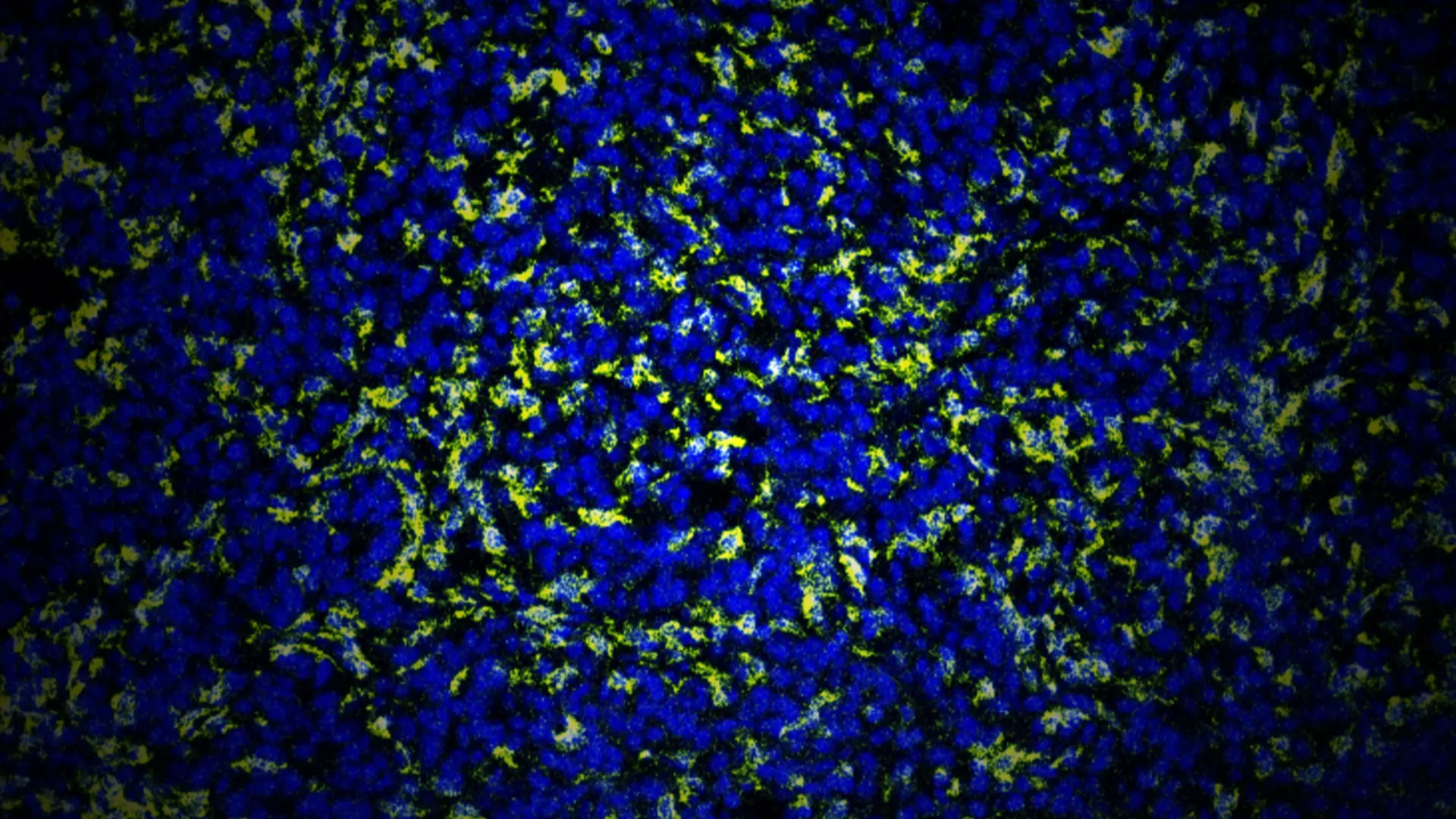
Control group, with macrophages highlighted in yellow
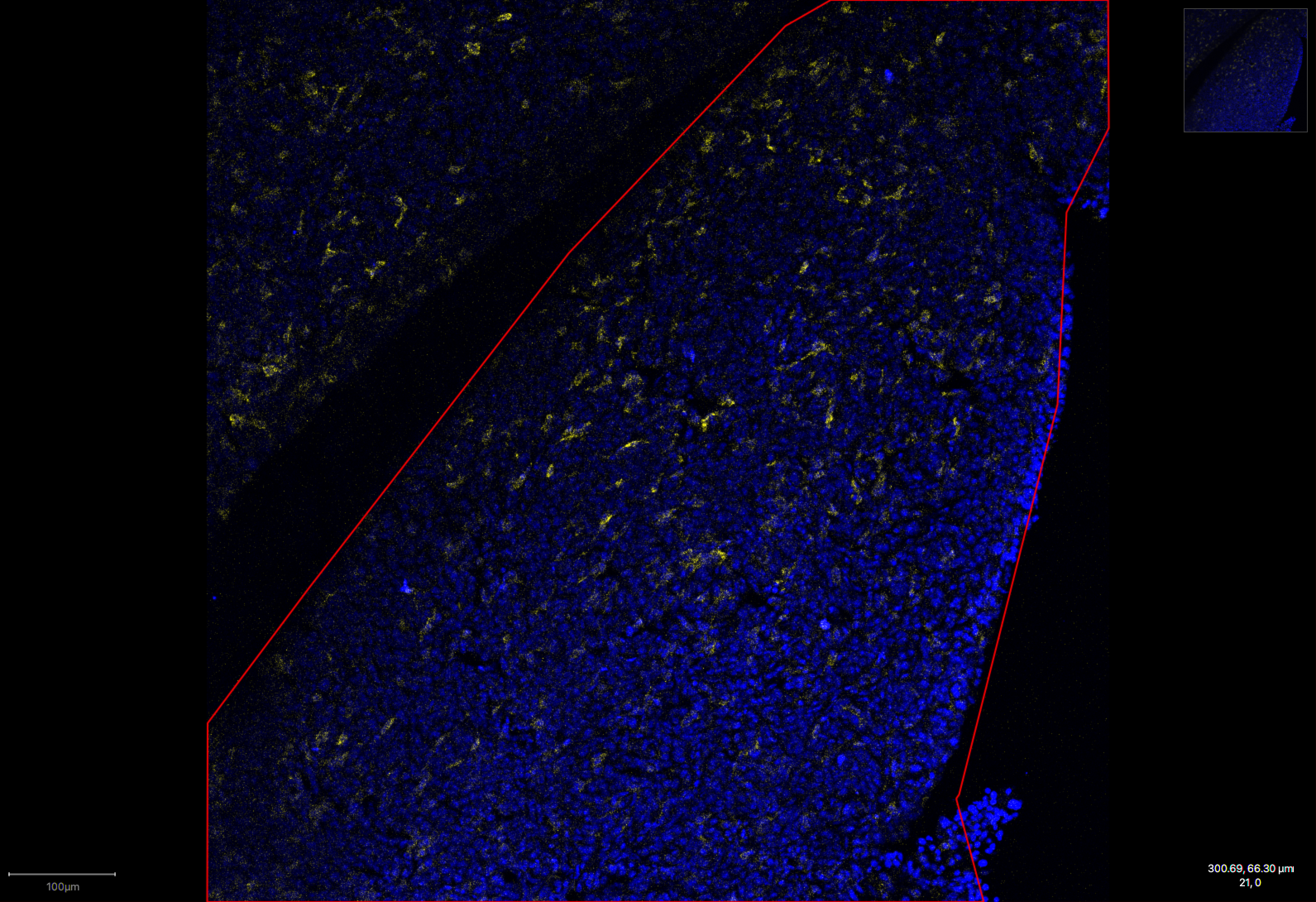
Treated group, demonstrating reduced macrophage levels
Using CAR-T Cells to Deliver Immune-Boosting Molecules Locally
To further improve immune responses to tumors, the team plans to take advantage of the very selective homing of the CAR-T cells and engineer them to act as “Trojan horses.” The CAR-T cells can be engineered to release more interferon gamma and other cytokines, chemokines, and bispecific antibodies that can supercharge the immune response within the tumor microenvironment, where they should have maximum effect with minimal damage to healthy tissue.
“Moving forward, we will test molecules that not only supercharge the T cells, but can also directly kill cancer cells, such as interferon alpha.”
- Brian Brown, PhD
Interferon alpha, a cousin of interferon gamma, was once considered a potential “magic bullet” for cancer because of its ability to kill cancer cells directly. However, it makes patients very sick when given systemically so it is no longer used as a cancer treatment.
A long-sought goal of cancer immunotherapy has been to deliver interferon alpha locally in the tumor. But this is not easy to achieve with metastatic diseases such as the cancers Dr. Brown and his team are working on.
The anti-macrophage CAR-T cells could be engineered to secrete interferon alpha to achieve local delivery of interferon alpha and other molecules into the tumor. “We have already established this with interferon gamma, so we know that our approach is capable of local delivery of an inflammatory cytokine directly in a tumor,” said Dr. Brown.
The Brown and Merad teams are now optimizing the design of their tumor macrophage-targeting CAR-T cells and generating humanized versions they hope will progress to the clinic.
The design of this new type of CAR-T cell builds upon several research endeavors in PrIISM that focus on understanding why specific macrophage and dendritic cell subsets can either dampen or promote tumor immunity.
To explore how different cancer cell genes control the immune cells in the tumor microenvironment in vivo and how they alter tumor growth and recognition of the cancer cells by the immune system, the Brown team has devised a first-of-its-kind spatial functional genomics screening platform called Perturb-map, described in a paper in Cell published in March 2022.
The Merad team also collaborated with Ido Amit, PhD, Professor of Immunology from the Weizmann Institute of Science in Israel to develop PIC-seq, a method to identify the genes that are switched on and off in cells that are physically interacting, which was described in a Nature Biotechnology paper published in March 2020. Using PIC-seq, the team uncovered key new details about the types of myeloid and T cells that influence responses to cancer immunotherapy with immune checkpoint blockade, and published the findings in Nature Cancer in March 2022.
Dr. Merad and her team continue to profile the different specialized populations of myeloid cells in the tumor microenvironment to find out how they operate at different stages of cancer, from tumor growth to metastasis. Together, these immune profiling and functional genomics studies will guide the rational design of other novel macrophage-targeting CAR-T cells and the payloads they should deliver to boost immune responses to cancer cells locally in the tumor microenvironment.

Team members of the Brown and Merad labs
Featured

Brian Brown, PhD
Director, Icahn Genomics Institute; Associate Director of PrIISM

Miriam Merad, MD, PhD
Mount Sinai Professor in Cancer Immunology; Director of PrIISM