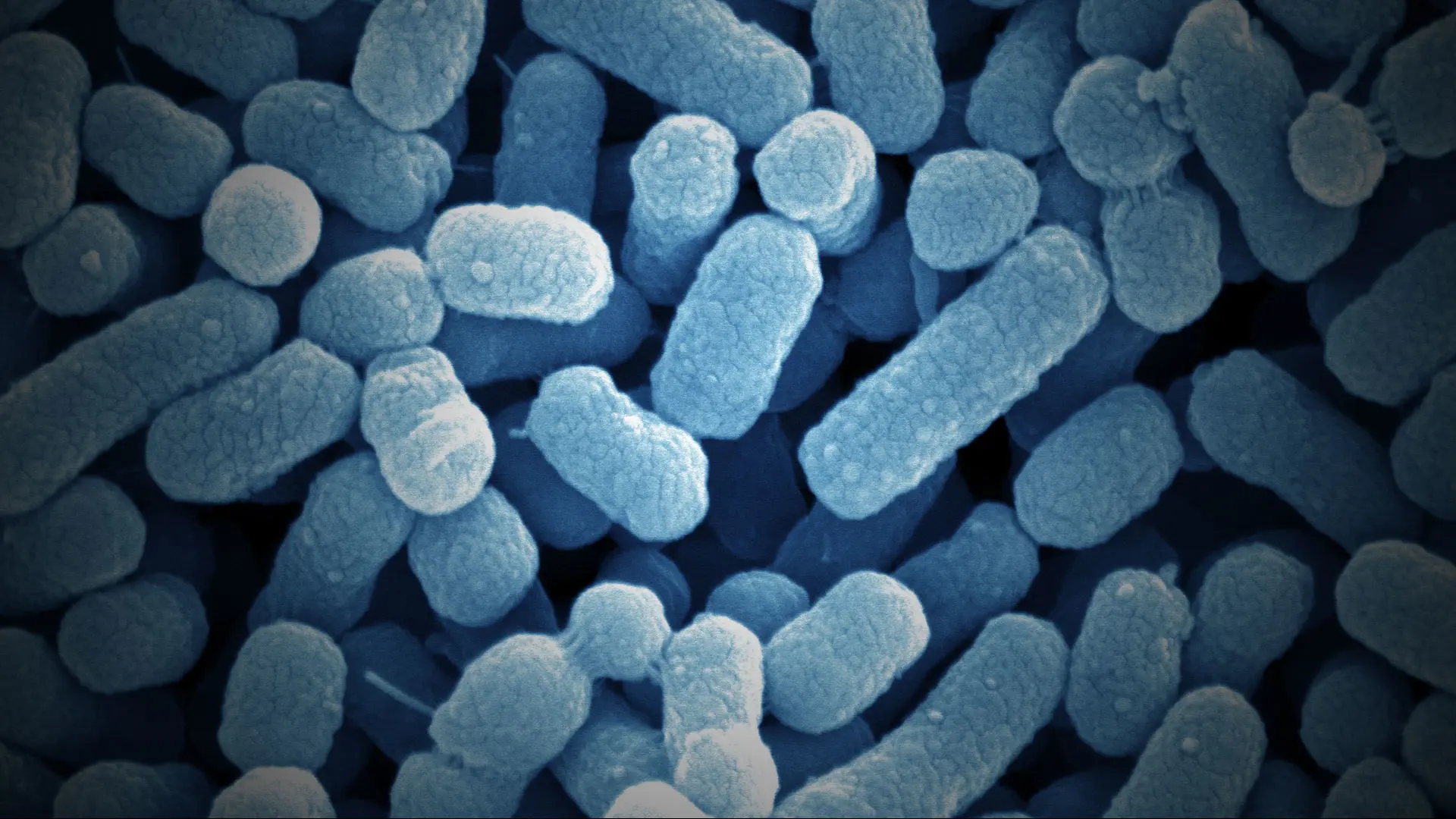
Severe C. difficile infection can be life-threatening, particularly in older people. With antibiotic treatment only working in around one in five patients, the current best therapy for recurrent C. difficile infection is fecal microbiota transplantation (FMT), where the gut microbiome of the patient is replaced with a stool sample from a healthy donor.
FMT is very effective, with a single transplant bringing the infection under control in up to 90 percent of patients. However, Jeremiah Faith, PhD, Associate Professor of Medicine (Clinical Immunology), and Genetics and Genomic Sciences, and co-Director of the Microbiome Translational Center at Mount Sinai, is keen to take the treatment one step further. He’s aiming to identify the key microbes responsible for the success of this procedure, so they can be manufactured for use as safe and standardized live biotherapeutics.
“We have the best chance of being successful with a synthetic version of a fecal transplant,” Dr. Faith said. The effort needed to create such a treatment is extensive: his team had to identify and purify the protective microbes and a very large bank of copies of the microbes had to be created to enable manufacturing at the scale needed for therapeutic use.
Dr. Faith’s team developed a new statistical tool called Strainer that can detect and track specific bacterial strains in metagenomic sequencing data from stool samples. Researchers were able to identify bacteria strains from FMT donors that successfully and consistently engraft into the recipients’ microbiome for at least five years, and which bacterial species were more likely to be found in C. difficile patients who benefitted from FMT. Results were published in Nature Microbiology in September 2021.
The live biotherapeutic under development contains 15 strains of protective bacteria, each grown individually under specific conditions applicable for human usage. “In the next month or so, we'll be combining them all together into a single live biotherapeutic that can be given to people,” said Dr. Faith. “Once we have this single ‘drug,’ we'll submit an Investigational New Drug Application to the U.S. Food and Drug Administration to propose studying this drug in the clinic.”
In addition to creating the synthetic transplant treatment, Dr. Faith’s team and the Microbiome Translational Center are also seeking to uncover the complex workings of the gut microbiome. This ecosystem has many millions of microbes that produce metabolites and first responder cells of the immune system that can move to other locations in the body. Understanding how this system works, in turn, sheds light on how the microbiome controls everything from inflammatory bowel disease to how different cancer patients respond to immune checkpoint inhibitors, supporting the ultimate goal of manipulating the microbiome therapeutically.
“We know that the mucosal immune system is very large and immune cells can go all over the body. But I always find this action potentially occurring at such a distance a little mind blowing.”
Jeremiah Faith, PhD
Fixing Faulty Microbiomes to Treat Multiple Sclerosis
Researchers at Mount Sinai have also been exploring the “gut-brain axis”—largely uncharted pathways connecting the gut microbiome to the brain. Stephanie Tankou, MD, PhD, Assistant Professor of Neurology at Mount Sinai, and her team have been delving into which bacteria in the gut microbiome can suppress or promote neuroinflammation in patients with multiple sclerosis (MS).
There had been extensive work backing a role for gut-brain axis communication in MS. A group in Germany showed that germ-free mice, which do not have any microbes in their guts, are resistant to the mouse equivalent of MS. Intriguingly, when microbes that are normally found in the gut are introduced to these germ-free mice, all of a sudden they are susceptible to the disease, said Dr. Tankou.
Additionally, a group at Dartmouth University showed that if regular mice were treated with a mixture of oral antibiotics, which got rid of at least 80 percent of the bacteria in their gut, those mice became resistant to MS-like disease.
“Clearly this tells you that susceptibility to these diseases is driven by gut microbes,” Dr. Tankou said. “This notion that we can control what's going on in the brain by manipulating the composition of the gut microbiome, sounded like something that came out of science fiction at first, and was too good to be true.”

Dr. Tankou’s work investigating the role of the gut microbiome in MS stretches back to 2014, when she was a MS fellow. She had shown for the first time that a probiotic can make the gut microbes composition of MS patients look like that of healthy subjects. She also found that the probiotic induced changes in the gut microbes composition of MS patients that were linked with decreased inflammation in the blood. The next step is to identify the beneficial gut microbes that are depleted in MS and turn them into drugs that can be tested in clinical trials to assess their efficacy.
There has been progress since, and Dr. Tankou’s team has mapped bacteria strains that are altered in the microbiomes of mice treated with the antibiotic vancomycin. In a paper published in October 2022 in Microbiome, the researchers showed findings that vancomycin protects mice from developing MS-like disease and this effect is driven by an increase in the abundance of beneficial bacteria in the gut.
Given these exciting findings, Dr. Tankou kicked off a funded pilot clinical trial to look at the effects of vancomycin on the composition of the microbiome, changes in peripheral immune cell function, and injury in the brain by magnetic resonance imaging, in 40 MS patients.
This is the first study examining the effect of short-term antibiotic use on the gut microbiome composition in MS patients. Dr. Tankou is hoping to see an increase in beneficial bacteria in the gut of vancomycin-treated patients, similar to the previous findings in mice. “And if we do see that, the nice thing is that we can actually isolate these ‘bugs’ from stool samples and further characterize them.” That would then set the stage for a hopeful future, where the isolated bacteria could be turned into probiotics for the prevention and treatment of MS and, hopefully, other central nervous system-related inflammatory and neurodegenerative diseases, she said.
Featured

Stephanie K Tankou, MD, PhD
Assistant Professor of Neurology

Jeremiah Faith, PhD
Associate Professor of Medicine (Clinical Immunology), and Genetics and Genomic Sciences; co-Director of the Microbiome Translational Center