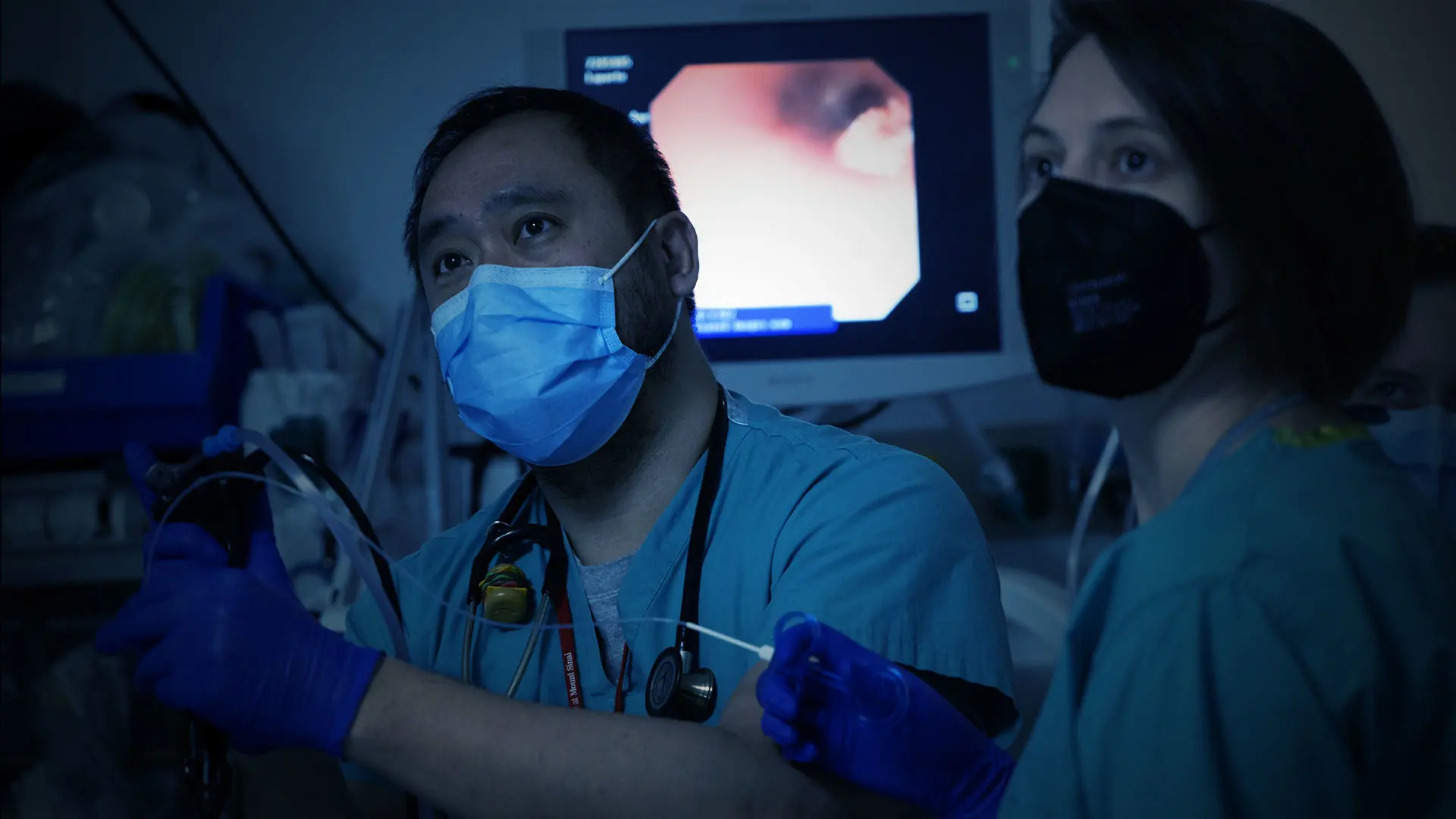
A new research program aims to map the developing pediatric airway from minimally invasive respiratory samples obtained during flexible bronchoscopy. The study, conducted by the Division of Pediatric Pulmonology at the Icahn School of Medicine at Mount Sinai, may eventually advance cystic fibrosis therapeutics and enable the minimally invasive diagnosis of other pulmonary diseases.
Single-cell RNA sequencing is a powerful tool that allows for the comparison of individual cellular transcriptomes through the identification of RNA molecules on a genomic scale and with high resolution. Single-cell RNA sequencing can thus be used to identify populations of cells within a sample or even transcriptional differences between corresponding cell populations in children with and without lung disease.
Supported by a Cystic Fibrosis Foundation Clinical Fellowship Award, physician-scientist Megan N. Januska, MD, Assistant Professor of Pediatrics, and Genetics and Genomic Sciences, at Icahn Mount Sinai, developed a research study leveraging this technology to define disease-related changes in the pediatric cystic fibrosis airway. She has identified unique gene expression profiles in immune cell populations obtained from children with cystic fibrosis and is now evaluating transcriptional changes in respiratory epithelial cell populations, which may help inform novel approaches to treatment. Although effective therapies designed to correct the malfunctioning protein in cystic fibrosis now exist for many people with the disorder, gaps in eligibility remain, due in large part to genetic heterogeneity. Indeed, the relative lack of CFTR modulator therapies available to children with cystic fibrosis from diverse backgrounds in the New York metropolitan area motivated Dr. Januska to pursue this research study.
More recently, Dr. Januska received a KL2 Scholars Award through ConduITS: the Institute for Translational Sciences at Icahn Mount Sinai, allowing her to expand the project outside the realm of cystic fibrosis. As Dr. Januska notes, “Understanding the cellular and molecular mechanisms that govern normal pediatric lung development and function will be critical to recognizing the dysregulation that occurs in disease.”
Alfin Vicencio, MD, Chief of Pediatric Pulmonology, is already envisioning potential clinical applications for the project. He says, “Applying these high-resolution investigative techniques to our already advanced diagnostic bronchoscopy program may enable minimally invasive diagnosis of rare and severe pediatric lung disorders in the future.”
Featured

Alfin Vicencio, MD
Professor of Pediatrics

Megan Januska, MD
Assistant Professor of Pediatrics, and Genetics and Genomic Sciences