Dr. Han discusses how recent discoveries in understanding the genetic characteristics of obesity hold vast potential in its control and treatment and how this will drive her future projects.
Q. What kinds of new approaches to the prevention and treatment of
obesity has your prior research at the National Institutes of Health (NIH) and
the University of Tennessee Health Science Center (UTHSC) opened up?
Dr. Han:
Understanding the genetic characteristics that underlie both the development of obesity and the body’s responses to obesity treatments holds tremendous opportunities for science and medicine to discover new ways to control and treat obesity, particularly in children because of their growth potential and inherent neuronal plasticity.
The focus of my laboratory’s research has been on elucidating the molecular mechanisms by which rare and common genetic variants alter metabolism and neurodevelopment. In my decade at the NIH, for example, my research group advanced clinical care models and
metabolic/neurocognitive research for patients with rare genetic disorders, like Alström, Bardet-Biedl, Prader-Willi, WAGR/11p deletion syndromes, and monogenic defects of the leptin signaling pathway.
Subsequently, during my time at UTHSC, our team established a cohort of children with obesity from the general population to study the interaction of common genetic variants and responses to weight-management therapies. In the laboratory, we have been using mice with these genetic defects to study precision medicine approaches for treating obesity.
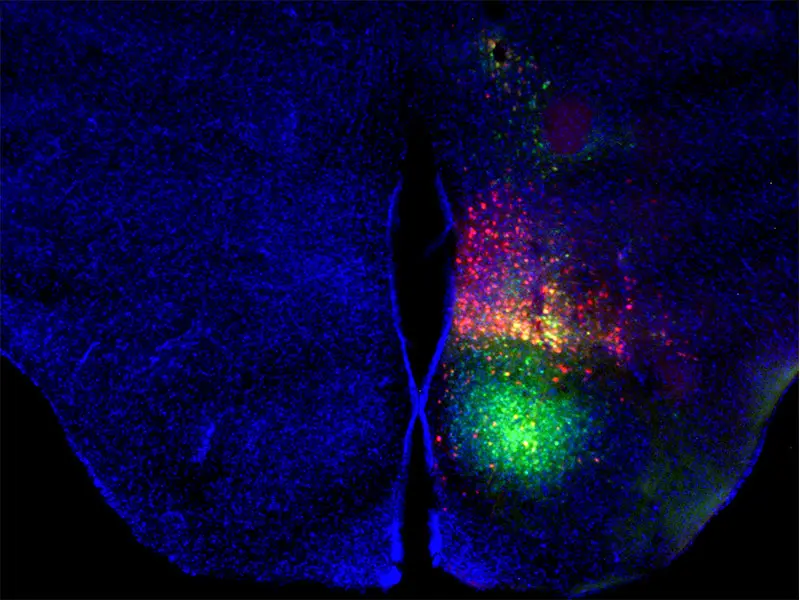
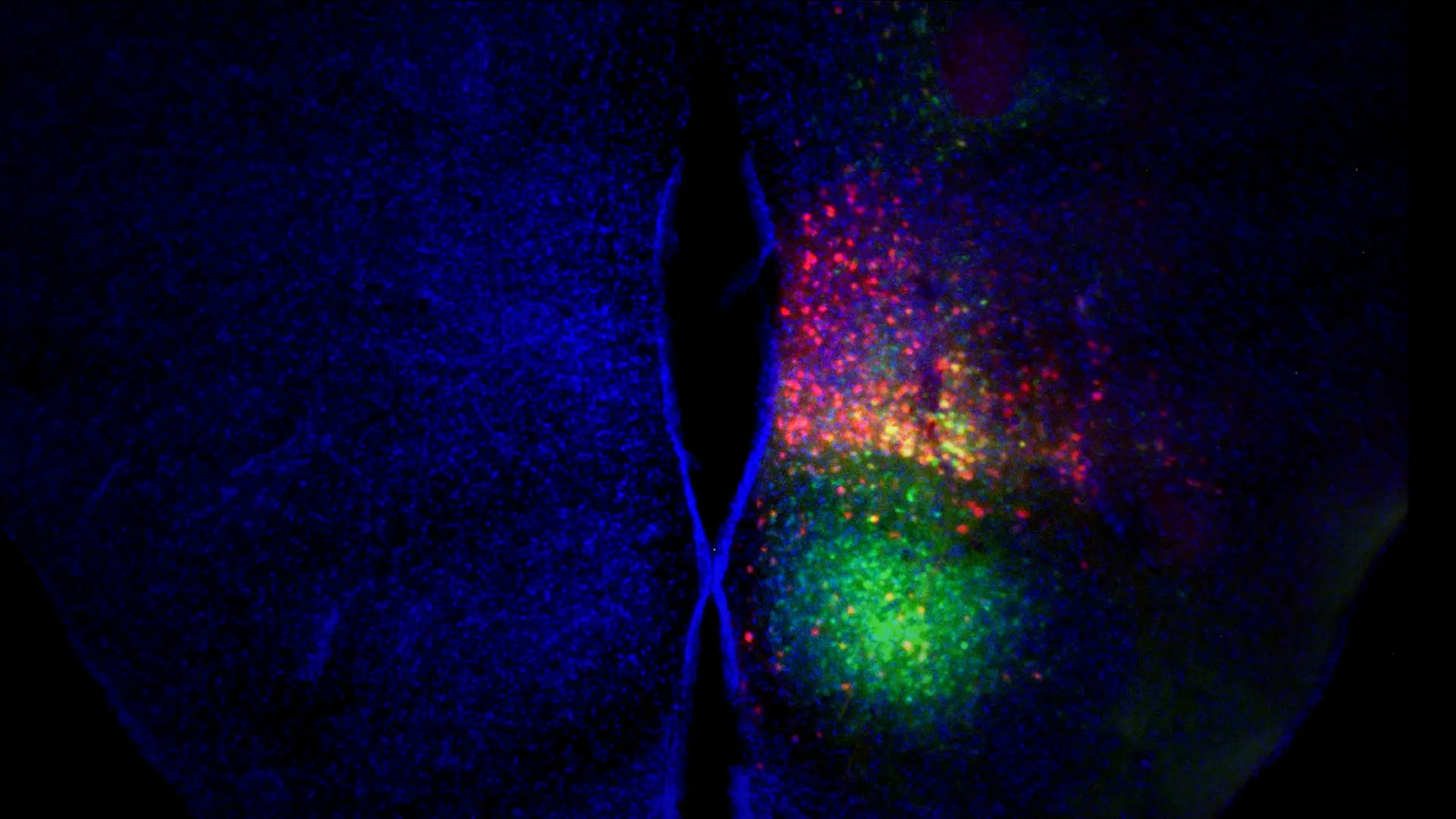
Image by Clint Kinney, PhD, Postdoctoral Fellow, The University of Tennessee Health Science Center.
Above: Mouse ventromedial hypothalamic nucleus after injection of a virus that expresses green fluorescent protein in all cells and a virus that expresses Cre-recombinase only in cells that express brain-derived neurotrophic factor from a specific promoter. Red = Cre recombination. Green = injection site. Blue = all cell nuclei to show tissue structure.
Q. What specific findings have you made regarding neuroendocrine
abnormalities of genetic variants associated with obesity?
Dr. Han:
Our most important finding has been the role of the brain-derived neurotrophic factor gene, or BDNF, in regulating appetite. We observed that patients with BDNF haploinsufficiency, a rare genetic condition in which one of the two copies of the BDNF gene is deleted, had significantly higher body mass index (BMI) and a fivefold greater risk of developing childhood obesity, confirming an important role of BDNF in human energy balance. These patients also had reduced pain perception and impaired intellectual and social development, indicating a role of BDNF in neurodevelopment, as well.
Our most important finding: the role of BDNF in regulating appetite.
Extending our findings from rare disorders to the general population, my team was able to show that common genetic variants of BDNF are associated with decreased hypothalamic BDNF expression and higher BMI, suggesting that genotype-specific therapies aimed at
augmenting BDNF signaling might be a precision medicine approach for treating obesity in patients with the variant gene. Using mice, we have actually identified distinct metabolic phenotypes associated with individual promoters for the BDNF gene that could serve as therapeutic targets.
Q. What have you learned about indices of insulin dynamics and obesity risk alleles in children that could inform future treatments?
Dr. Han:
While obesity is a well-established factor in developing insulin resistance, the role of insulin resistance in future weight gain in children is not well understood. My lab examined the association between energy intake at a standard buffet lunch and indices of insulin dynamics in children with obesity. We observed that insulin resistance and hyperinsulinemia predicted higher caloric intake, suggesting that these factors might predispose someone to further excess weight gain. Just as importantly, we found that interventions aimed at improving insulin sensitivity could serve as valuable adjuncts to weight management in children with obesity.
“We’re even looking at newer approaches, such as CRISPR activation to target particular genes in specific brain regions...”
- Joan Han, MD
Q. How does your lab intend to build on these important findings at your new home within Mount Sinai and Mount Sinai Kravis Children’s Hospital?
Dr. Han:
Ultimately, I think that obesity therapy individualized to each patient will be far more
effective than a one-size-fits-all approach. Therefore, my research group’s focus is on identifying novel treatments that range from specialized diets and exercise programs to microbiome modulators and precision medications. We’re even looking at newer approaches, such as CRISPR activation to target particular genes in specific brain regions in order to suppress appetite and increase energy expenditure while avoiding, or at least minimizing, off-target side effects.
What attracted me to Mount Sinai were the incredible clinical and basic science resources—particularly in genomics, neuroscience, and metabolism—that align perfectly with my interests. I am grateful for the support of the Department of Pediatrics, and the Diabetes,
Obesity, and Metabolism Institute, and look forward to many fruitful collaborations across the Mount Sinai Health System campus.
Featured

Joan Han, MD
Professor and Chief, Division of Pediatric Endocrinology and Diabetes, Mount Sinai Kravis Children’s Hospital