During his long and prestigious career as a hand surgeon, Michael R. Hausman, MD, Chief of Hand and Upper Extremity Surgery for the Mount Sinai Health System, has worked to solve an enduring dilemma: Surgery to repair damaged peripheral nerves is most likely to produce the best outcomes if it is performed close to the time of injury, yet it can take six months to a year before it becomes clear whether a nerve will heal on its own or require neurolysis, nerve repair, or nerve graft.
“Currently, we have no way to predict which nerves will recover and which will not,” says Dr. Hausman, the Dr. Robert K. Lippmann Professor of Orthopaedics and Vice Chair, Department of Orthopedics, Icahn School of Medicine at Mount Sinai.
With a breakthrough discovery published in a front-page article in Clinical Orthopaedics and Related Research in June, Dr. Hausman; Paul J. Cagle, Jr., MD, Associate Professor, Orthopedic Surgery, Icahn School of Medicine; and Christoph A. Schroen, a doctoral student at Heidelberg University – BG Klinik Ludwigshafen, Germany, and a predoctoral research fellow at the Icahn School of Medicine at Mount Sinai, who works in Dr. Hausman’s lab, are now closer than ever to solving this problem. They have identified a new method they believe could predict, soon after injury, whether a damaged peripheral nerve is likely to recover without intervention. In the process, they uncovered new findings about the sequence of nerve damage that transforms the traditional paradigm of peripheral nerve injury.

From left: Michael R. Hausman, MD; Christoph A. Schroen, predoctoral research fellow; Paul J. Cagle, Jr., MD; and Leesa Galatz, MD, MBA, Mount Sinai Professor and Chair, Department of Orthopedic Surgery, Icahn School of Medicine at Mount Sinai
Nerve Injury and Imaging: Developing a New Research Model
To study the problem of nerve recovery, Dr. Hausman and his colleagues first needed to identify a reliable and reproducible model for injuring nerves. They developed a new functional measurement method that applies force to stretch median nerves in the forelimbs of anesthetized rats. Then, Dr. Hausman and his team used dual-photon microscopy—a specialized type of fluorescence microscopy that uses near-infrared light for deep, noninvasive penetration into tissue—to image the nerves and assess the damage.
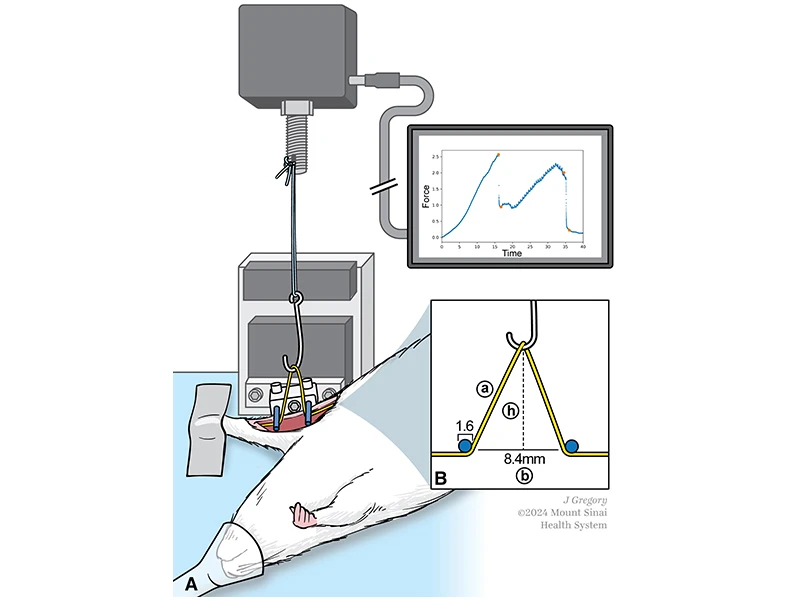
This illustration shows the experimental setup used to apply stretch to the median nerve in vivo. (A) A hook attached to a load cell stretches the median nerve of an anesthetized rat between two hold-down pins, while force-time curves are generated in real time. (B) Nerves are approximated to form an isosceles triangle during stretching, with “a” depicting one side of the triangle, “h” depicting the hook’s height, and “b” indicating the distance between both pins.
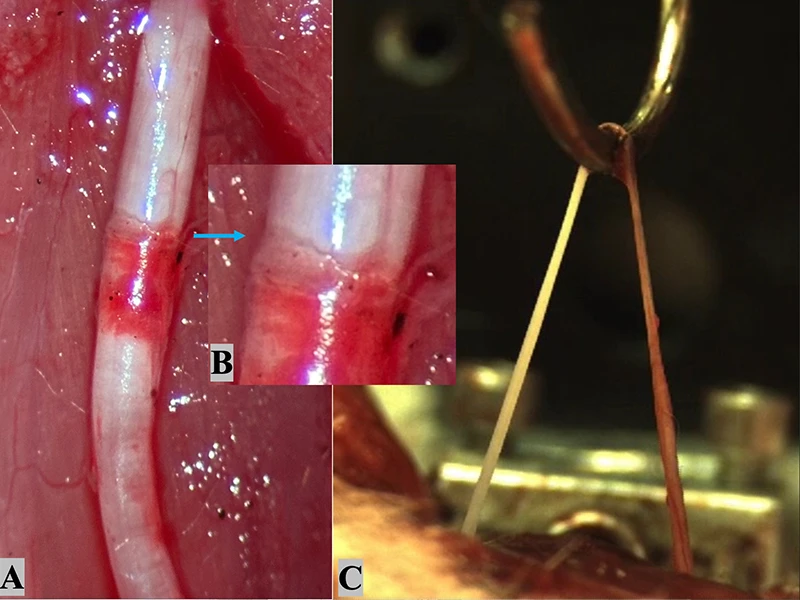
(A) After epineuroclasis, rupture of the epineurium and a subepineurial hematoma are visible with an exposed nerve core proximal to the epineurial tear zone (image taken with a smartphone camera). (B) A magnified view of the epineurial tear zone reveals the sharp edge of the disrupted epineurial sheath. (C) Macroscopic continuity is maintained immediately after endoneuroclasis (screenshot from filmed load-to-failure experiment). The exposed nerve core (left of the hook) appears white, whereas the area with an epineurial sheath (right of the hook) shows a red color.
“This second-generation microscopy allows us to look into the substance of the nerve to see what’s going on with the internal wiring,” Dr. Hausman says. “This technology had never been applied to nerves before, and we realized it could allow us to image the nerves in a nondestructive way—performing something such as a biopsy without cutting the nerve tissue.”
Applying the imaging technology, the researchers compared the physical damage in rat nerves that had experienced varying degrees of force. They also tested the amount of electricity required to stimulate each damaged nerve. The study led to a surprising discovery. It had long been assumed that when a nerve is stretched, the endoneurium would rupture before the epineurium.
“In fact, we found it was the opposite—the external layer ruptured first,” Dr. Hausman says. “It turns out that the epineurium is the weak link in the chain.”
Classifying Nerve Stretch Injuries
Discovering this “outside-in” sequence of nerve damage points toward a new method for classifying the severity of an injury: the “Neuroclasis Classification,” which is based on the order in which different parts of a nerve rupture. The researchers discovered that damage to the outermost tissues alone could impair nerve conductivity, but the functional impairments were even more significant when the damage extended into the endoneurium, as well.
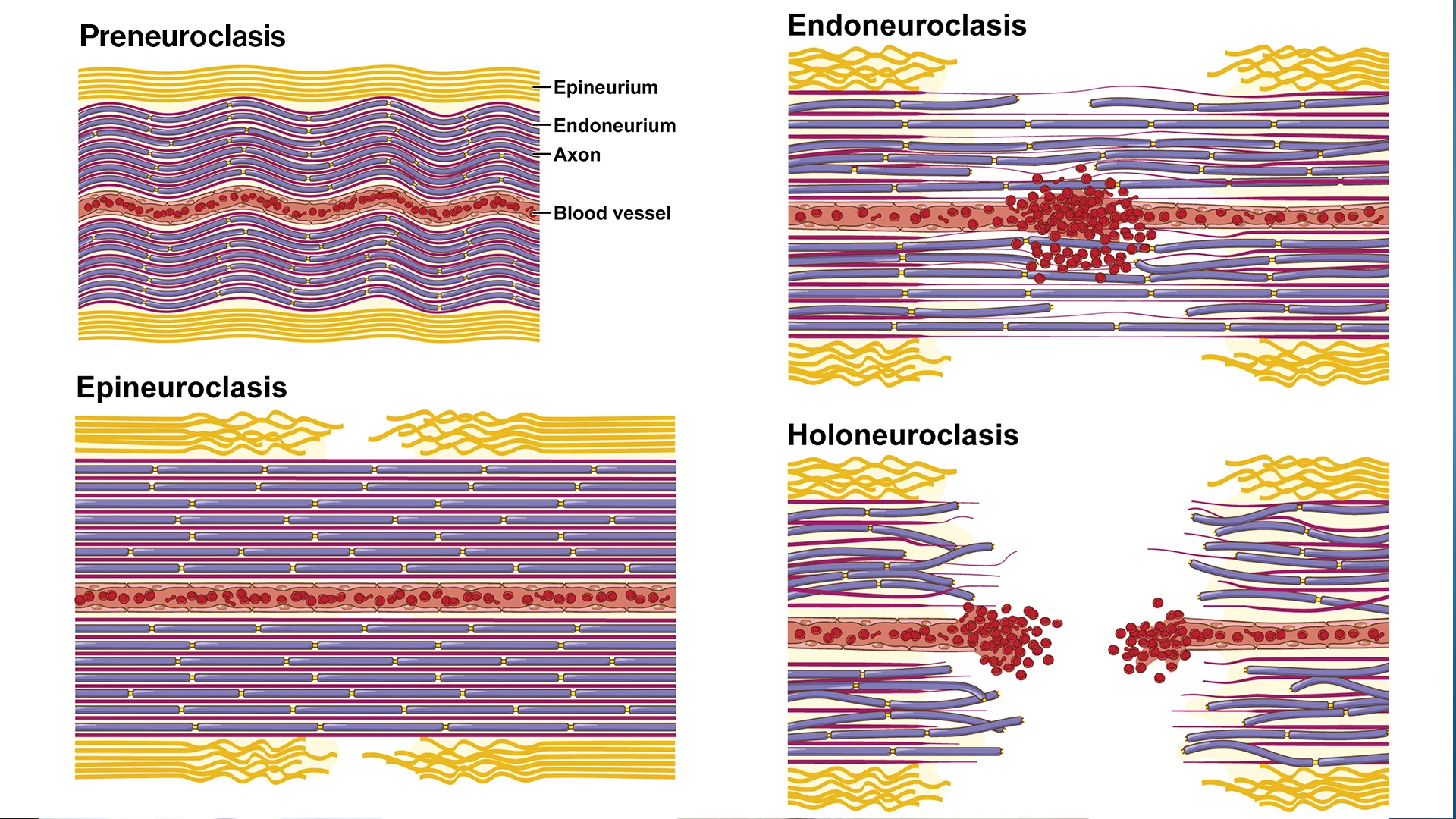
Illustration showing the four levels of neuroclasis: preneuroclasis, epineuroclasis, endoneuroclasis, and holoneuroclasis. The rat median nerve follows an "outside-in" sequence of structural failure, starting at the epineurial sheath and ending at the axonal level.
The finding was revelatory: Patients with damage to the inner nerve tissues may be most likely to benefit from surgical intervention.
“Nerves that are badly injured may still have some capacity to recover,” Dr. Hausman says. “This finding will help us identify, soon after injury, which injured nerves will recover on their own and which will require surgery.”
Dr. Hausman and his colleagues are continuing to study nerve damage using their new model of nerve stretch injuries. Rodents heal better than humans from peripheral nerve injuries, so more work is needed to validate the findings in large animal models and, eventually, to verify that nerve injuries follow similar patterns in humans.
These findings could have important implications for the development of new diagnostic tools. Dr. Hausman envisions a diagnostic procedure such as a virtual biopsy that would identify internal nerve damage without removing tissue. Such a procedure would likely require an incision to access the damaged nerve. Then, a surgeon could use a probe to assess the damage using dual-photon microscopy. “It would require a surgical procedure, but not a destructive one,” he says.

Drs. Cagle (left) and Hausman (right)
While that procedure does not yet exist, the findings bring Dr. Hausman and his colleagues closer to their goal of identifying which patients would benefit from surgery early on, when interventions are most likely to be effective. This could affect care for patients with peripheral nerve injuries.
“In any sort of extremity injury, the rate-limiting step for recovery of function is the nerve. We can repair a broken bone. If blood vessels are damaged, it is relatively simple to fix the plumbing, but we are not very good electricians,” Dr. Hausman says. “If this all works out the way we envision, it would be an enormous step forward to helping people with severe injuries of the arms and legs.”
Featured

Michael R. Hausman, MD
Professor of Orthopedic Surgery

Paul J. Cagle, Jr., MD
Associate Professor of Orthopedic Surgery

Christoph A. Schroen
Predoctoral Research Fellow, Icahn School of Medicine at Mount Sinai
