A new study led by Mount Sinai’s Woojin Han, PhD, is addressing one of the biggest and oldest challenges in managing patients with rotator cuff injuries—the subsequent risk for fatty degeneration of the adjacent shoulder muscle, which is often associated with high re-injury rates.
“It is a very significant problem, given that approximately one-third of all adults in the United States could experience a rotator cuff injury due to sports or age-associated wear and tear,” says Dr. Han, Assistant Professor, Orthopedics; Orthopedic Research; and Cell, Developmental and Regenerative Biology, Icahn School of Medicine at Mount Sinai. “However, there is nothing in the clinical space that is available to treat or prevent skeletal muscle degeneration.”
Dr. Han aims to fill that gap with an innovative approach to treatment. His lab, the Han Laboratory for Cell-Instructive Biomaterials and Regenerative Engineering, which is focused on technologies that promote musculoskeletal soft tissue regeneration, has received an FY23 Applied Research Award from the Department of Defense to develop an injectable protein-releasing hydrogel patch that has the potential to prevent, or even reverse, rotator cuff muscle degeneration.
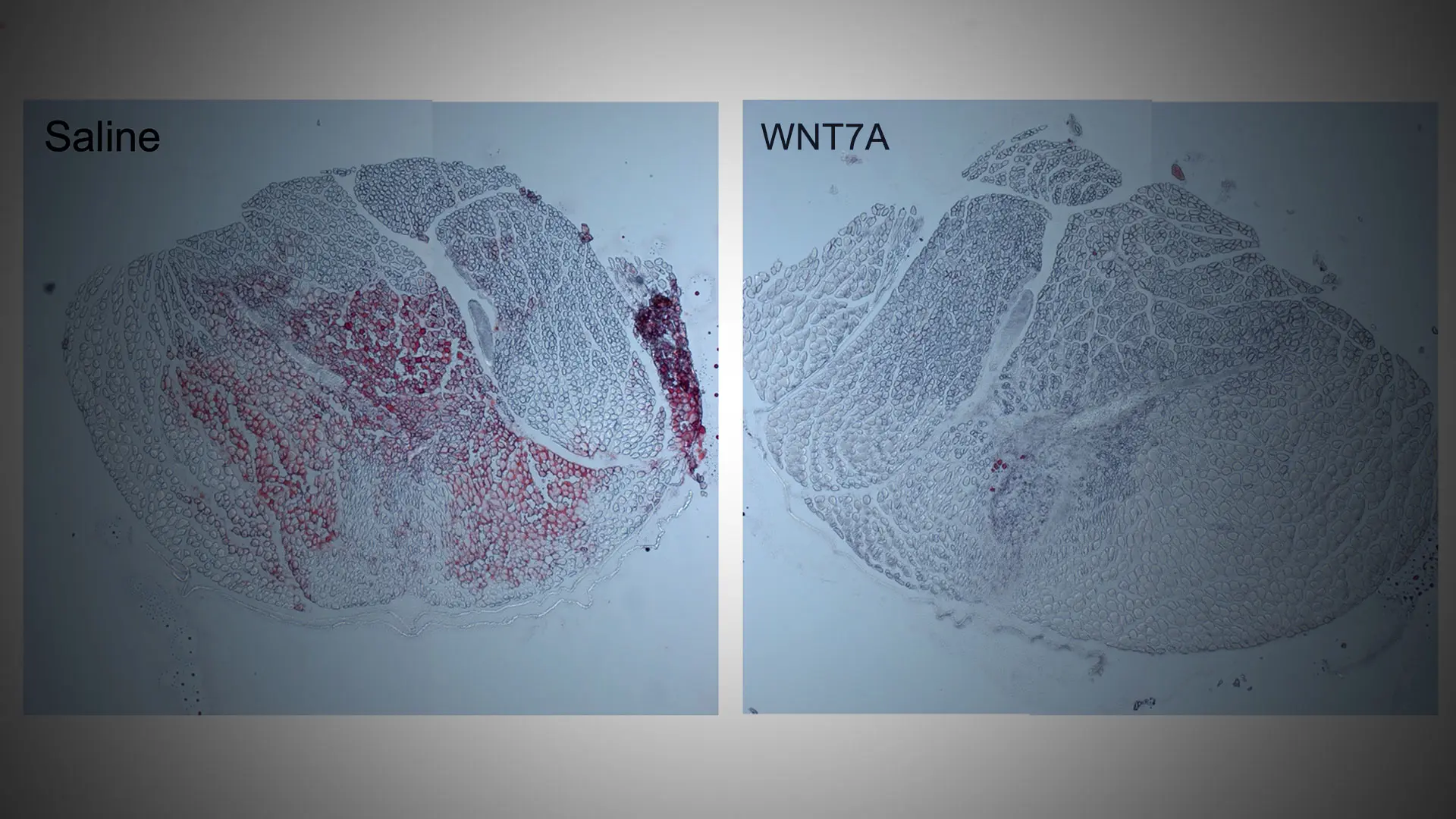
The hydrogel patch will steadily release a protein called WNT7A, which plays a key role in embryo development and homeostasis of skeletal muscle. Previously, Dr. Han conducted a study demonstrating that, when delivered using a hydrogel patch, this protein has the ability to simultaneously reduce fatty infiltration and muscle wasting, which suggests therapeutic potential for rotator cuff muscle disease. The results of his study, “WNT7A suppresses adipogenesis of skeletal muscle mesenchymal stem cells and fatty infiltration through the alternative Wnt-Rho-YAP/TAZ signaling axis,” were published April 11, 2023 in Stem Cell Reports.
“We have isolated endogenous muscle cells that turn into adipocytes or fat cells in the skeletal muscle in a dish and successfully treated them with the WNT7A protein to prevent them from turning into adipocytes,” Dr. Han says. “We also found that it worked in vivo, but the models we worked with do not reflect what happens in a clinically relevant injury model, which is what we are assessing now.”
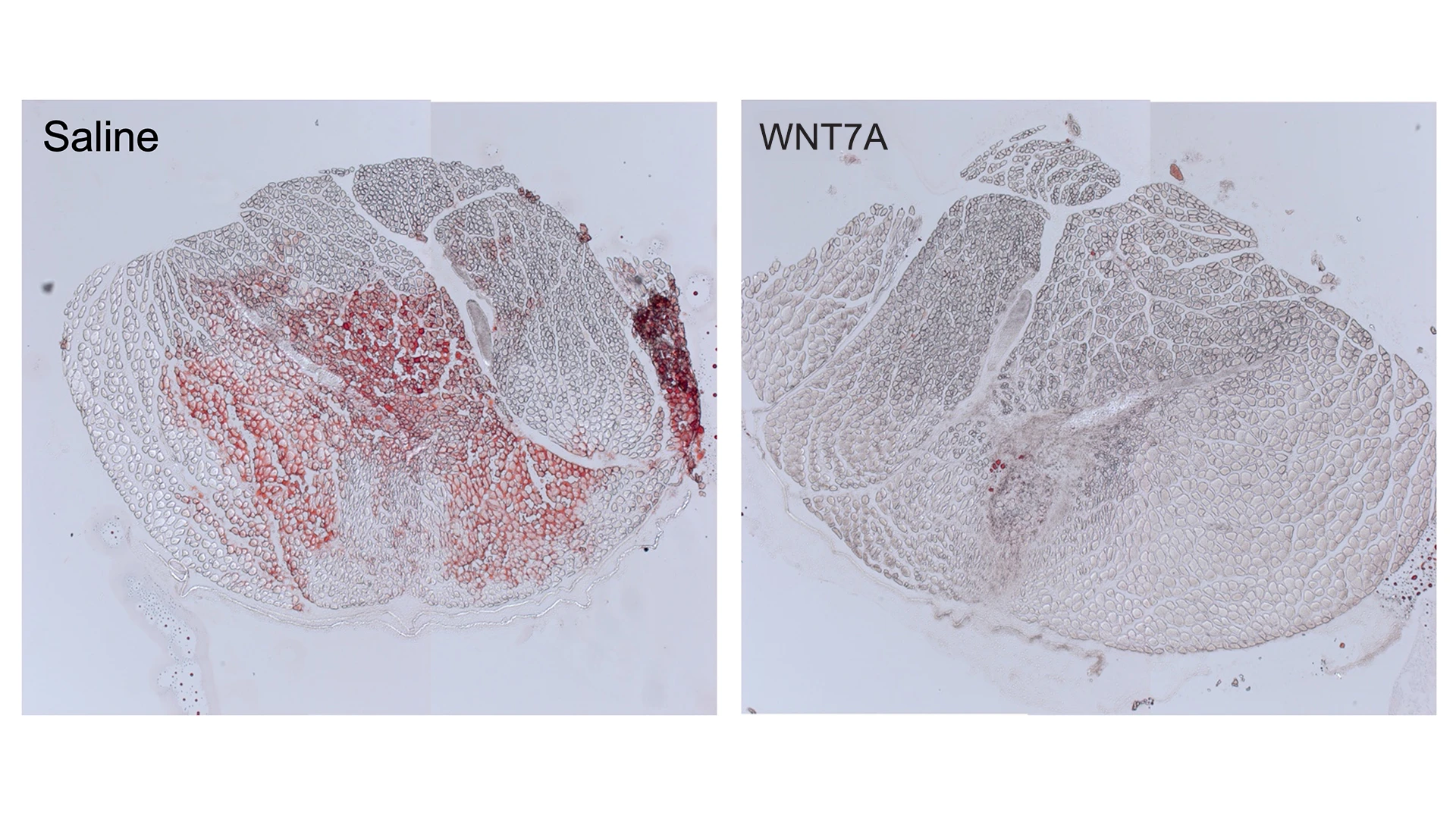
WNT7A injection significantly reduces intramuscular fatty infiltration in a proof-of-concept mouse model. Red (Oil Red O) staining indicates fat.
Preventing and Reversing Muscle Degeneration
In his upcoming study, Dr. Han is using mouse models to simulate rotator cuff injuries and deliver the protein-releasing hydrogel therapeutic to evaluate its potential for preventing muscle degeneration and, at a later point, to see whether it reverses degeneration. He believes delivering the protein using the synthetic hydrogel, which he developed during his postdoctoral fellowship training at the Georgia Institute of Technology, will result in long-term treatment with enhanced regenerative outcomes. Such results, he notes, are not possible using traditional intramuscular injections, which are associated with high-dosage requirements, rapid clearance, instability, and short half-life. This could be beneficial for individuals at high risk for rotator cuff injuries, such as active-duty service members.
“Among the military population, the number of individuals with rotator cuff injury is larger because of the nature of their work,” Dr. Han says. “There is also a significant percentage of veterans who are discharged due to shoulder problems. That is why the military is interested in this study because it has the potential to enhance combat readiness and skills and decrease medical discharges.”
The priority for Dr. Han in this three-year study is to determine optimal dosage and release of the WNT7A protein based on assessments of muscle growth, quality, and contraction as well as observed gait patterns among mice models. He is planning further animal model studies before proceeding with human clinical trials.
“This will not replace surgical intervention,” Dr. Han explains. “The goal is to produce a therapeutic that could be used with surgery to prevent progressive degeneration and reduce reinjury risk or to promote regeneration in addition to adjacent treatments. If we can achieve that, we can significantly reduce pain among patients who have experienced muscle degeneration and improve their quality of life.”
Featured

Woojin Han, PhD
Assistant Professor, Orthopedics; Orthopedic Research; and Cell, Developmental and Regenerative Biology