New York Eye and Ear Infirmary of Mount Sinai (NYEE) was active in oculomics well before it became an industry buzzword and area of interest around 2020. We are now poised to be a major player in the field through our groundbreaking work with optical coherence tomography angiography (OCTA) and adaptive optics scanning light ophthalmoscopy (AOSLO) to image the retinal capillary bed and thereby measure the activity and severity of sickle cell disease. These technologies also measure the response of patients to treatment, including the first CRISPR-based gene editing regimen approved by the U.S. Food and Drug
Administration (FDA) in December 2023 for sickle cell disease, signaling a huge advance in genome editing technology.
Sounding the Alarm on Signs of Sickle Cell Disease
This is welcome news to more than 100,000 Americans, most of them of African descent, who experience sickle cell disease, which causes red blood cells to become hard, sticky, and crescent shape, blocking blood flow to the rest of the body.
“Many conditions of the eye give us early clues about the presence of other systemic diseases in the body—including diabetes, cardiovascular, hypertension, sickle cell, and Alzheimer’s—that might otherwise go undetected,” says Richard B. Rosen, MD, Vice Chair and Director of Research at NYEE, and the Belinda Bingham Pierce and Gerald G. Pierce, MD, Distinguished Chair of Ophthalmology at the Icahn School of Medicine at Mount Sinai. “The ability of our advanced imaging technology to study intermittent perfusion events in sickle cell disease clearly shows the potential of oculomics, while giving us an advantage in this important space.”
The eye as a harbinger of a wide range of pathologies gained credence with the work of R. Theodore Smith, MD, PhD, Director of Biomolecular Retinal Imaging at NYEE. His lab identified a strong association between subretinal drusenoid deposits found above the retinal pigment epithelium in age-related macular degeneration (AMD) and systemic vascular disorders impacting the heart, including coronary artery disease, cardiac valvular disease, hypertension, and stroke. That revelation is meaningful for ophthalmologists and cardiologists alike, who now have evidence to justify cross-treating patients who show either AMD or heart disease.
Coupling advanced imaging with machine learning is now allowing clinicians for the first time to watch sickle red blood cells flow through the capillaries and visualize the mechanisms of vaso-occlusion.
“We noticed that capillaries would open and close momentarily, an early sign of vaso-occlusion, which can result in blindness if blood flow blockage is prolonged,” explains Toco Y.P. Chui, PhD, Director of the David E. Marrus Adoptive Optics Imaging Laboratory at NYEE.
That observation led Dr. Chui to develop the Intermittent Perfusion Index, an algorithmic-driven measure of what percentage of capillaries in the retina are transiently blocked over the course of an hour, based on repeat or sequential imaging during that timeframe.
In a study published in Case Reports in Hematology, Dr. Chui and research colleague Jeffrey Glassberg, MD, MA, Director of the Mount Sinai Comprehensive Sickle Cell Program, found the OCTA/Intermittent Perfusion Index approach to be an effective biomarker for measuring the severity of sickle cell disease, as well as the efficacy of novel CRISPR-based gene editing.
NYEE plans to add to that sophisticated tool set a new AOSLO capability that can measure the blood flow velocity within single capillary segments, giving clinicians a more granular view of disease progression and response to therapy.
“Following the FDA’s recent approval of gene therapy for sickle cell disease, we look forward to seeing more patients who are cured of the disease and to better understand their recovery. There are a wide range of additional vascular, neurologic, and metabolic conditions that may soon be treatable with similar gene therapy approaches,” notes Dr. Rosen. “Clinical cellular imaging will be an important tool for assessing an individual’s response to therapy and will pave the way toward advancing the adoption of gene therapy as a standard clinical solution.”
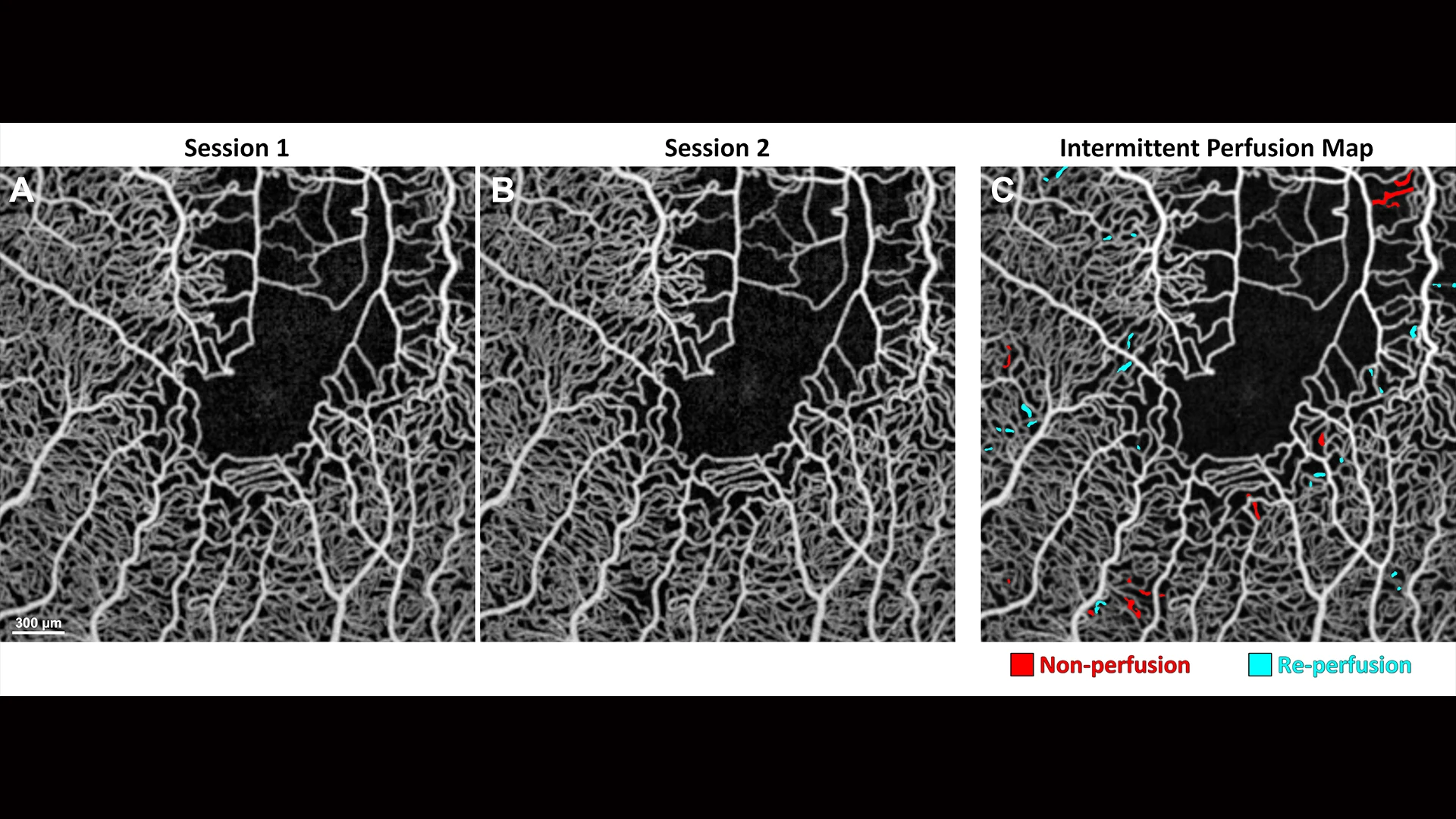
Averaged OCT angiography images of a sickle cell disease patient collected at (A) baseline and (B) one-hour follow-up. (C) Intermittent perfusion map shows capillary non-perfusion in red and re-perfusion in cyan over one hour.
New Evidence Cites Reduced Ocular Perfusion as a Mechanism for Macular Degeneration
Scientists have sought for 30 years to make the connection between age-related macular degeneration (AMD) and systemic vascular disease, which includes myocardial infarction and ischemic stroke. A group of recently published studies from researchers at NYEE makes that association tighter than ever through strong evidence that vascular disorders compromise the flow of blood (or perfusion) to the retina, in turn serving as a mechanism for the formation of subretinal drusenoid deposits (SDDs), telltale signs of age-related macular degeneration. Just as importantly, the new findings make a strong case for using SDDs as highly sensitive biomarkers for not just AMD, but vascular pathologies that might otherwise go unrecognized.
“The public health message from our studies couldn’t be more clear: if SDDs are detected, especially in patients with cholesterol abnormalities, they should trigger a cardiovascular consult for the patient to detect potential high-risk vascular diseases, and potentially save lives,” says Dr. Smith, senior author of the studies. “Likewise, widespread screening of patients with known vascular disorders for SDDs could lead to the discovery of AMD, and potentially save their sight.”
The research team advanced another mechanism for the SDD phenotype: genetic risk and, more specifically, the ARMS2 gene, which provides instructions for making a protein found in light-sensing tissue in the retina. “We showed that HARM2, as we renamed a double dose of the harmful form of ARMS2, is a second major risk factor for SDDs independent of high-risk vascular disease,” notes Dr. Smith. “While more research is required, recognizing this gene’s central role in AMD could position it as a future target for therapeutic interventions.”
Following is a more detailed look at the three NYEE studies that could open an important new chapter in diagnosing and treating macular degeneration by navigating promising cardiovascular pathways.
The Carotid Artery Connection
In their effort to determine if ocular hypoperfusion from internal carotid artery stenosis is associated with subretinal drusenoid deposits, scientists relied on a perfect experimental system handed to them by nature. They learned from evaluating ischemic stroke patients that moderate or greater blockage of the carotid artery was strongly associated with SDDs and thinning of the choroid—the vascular membrane that lies between the retina and the sclera—on the same or ipsilateral side of the body. Since the unblocked side was unaffected, this pathology offered convincing proof that downstream ophthalmic artery and choroidal hypoperfusion from internal carotid artery stenosis was a mechanism for SDD formation.
As reported in the February issue of Investigative Ophthalmology and Visual Science, that research is the first to demonstrate a significant association between increasing ICA stenosis and ipsilateral SDDs. Investigators concluded that additional research is warranted to determine whether using optical coherence tomography imaging to detect SDDs could serve as an inexpensive mass-screening tool for high-risk vascular disorders, potentially leading to life-saving evaluations for patients.
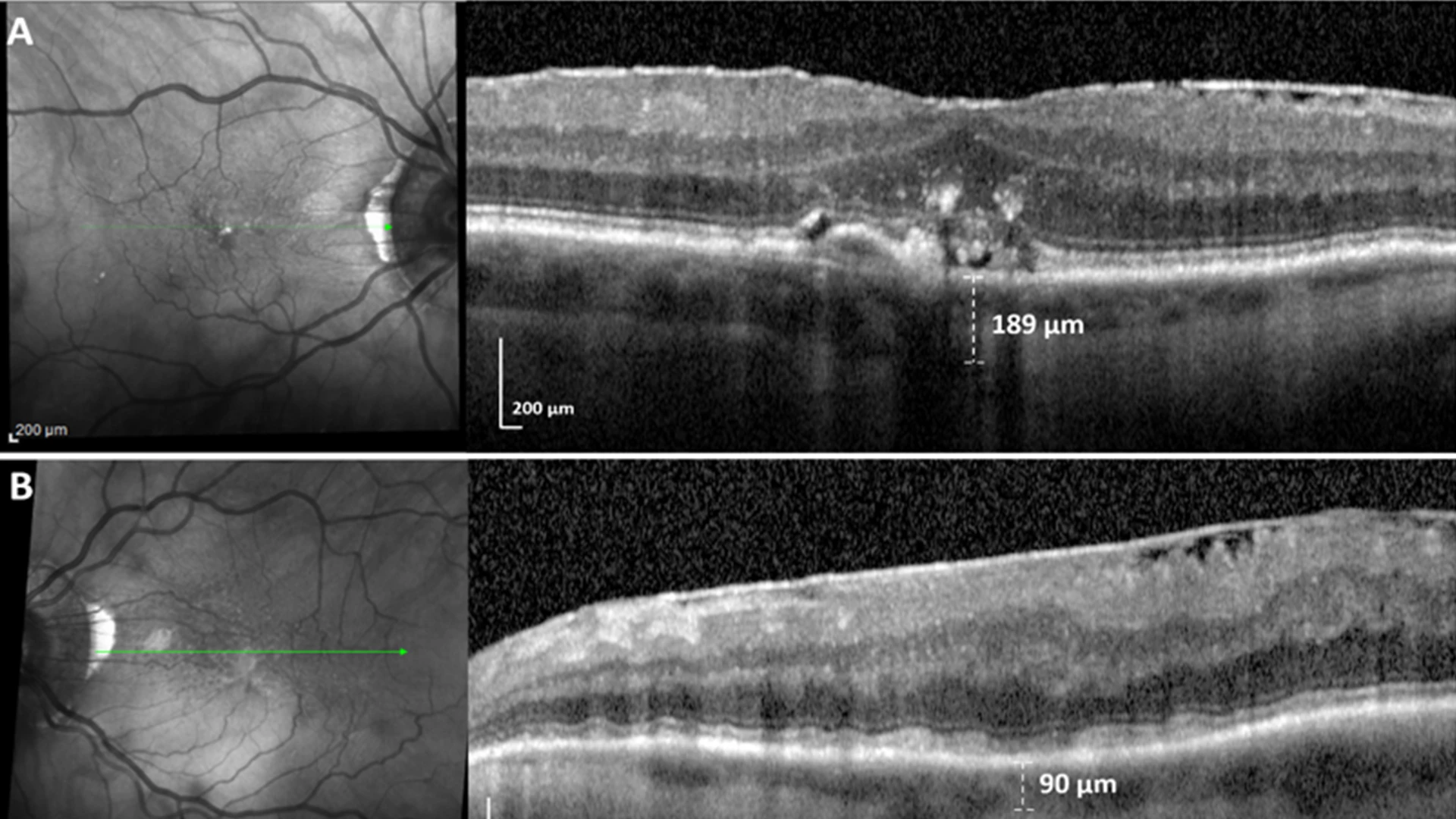
Unilateral SDDs caused by a dose response to unilateral internal carotid artery (ICA) stenosis.(A) SD-OCT scan, right eye, downstream of mild right ICA stenosis, shows no SDDs and a few drusen. (B) SD-OCT scan, left eye, downstream of moderate left ICA stenosis, shows confluent SDDs and choroidal thinning significantly associated (p=0.002, p=0.008) with ipsilateral ICA stenosis in a dose response to moderate (> 50%), not mild, ICA stenosis.
Linking SDDs to Thinning of the Photoreceptors
Age-related macular degeneration is known to damage the ellipsoid zone of the photoreceptors, the cellular components of the retina that convert light entering the eye into signals that are transmitted to the brain. In this study, published in the February issue of BMJ Open Ophthalmology, NYEE researchers demonstrated in their cohort of patients with AMD a quantitative link between SDDs and decreased ellipsoid zone thickness.
This major finding, they emphasized, is consistent with and offers strong evidence that ellipsoid zone thinning is a critical marker of the reduced and insufficient ophthalmic perfusion that causes SDDs and, therefore, hypoxic photoreceptor damage in cases of high-risk cardiovascular disease and stroke. Clinicians, they concluded, could provide enhanced multidisciplinary care to their retinal and cardiovascular patients by better understanding this emerging link between AMD and systemic vascular disease.
Decreased Perfusion From Valvular Heart Disease
This study’s model of SDDs in age-related macular degeneration draws a straightforward line to valvular heart disease, particularly mitral and aortic valve disorders, which affect about 2.5 percent of the U.S. population, rising to 13 percent in those over 80. And as in their companion papers, researchers learned that SDD deposits were significantly more common with valvular heart disease of moderate severity or greater, once again supporting a proposed mechanism of SDD formation through a cardiovascular, then an ocular, perfusion deficit.
In a paper published in the March issue of European Journal of Ophthalmology, scientists said their findings implied that an ophthalmologist detecting SDDs should promptly refer the patient to a cardiologist for a standard workup for vascular disease. The urgency is enhanced in patients who also have low levels of HDL, the “good” cholesterol that protects against vascular disease. Likewise, the presence of mitral or aortic valve disease should trigger a workup by an ophthalmologist for SDDs and AMD as part of a public health effort for early detection of potentially blinding and life-threatening diseases.
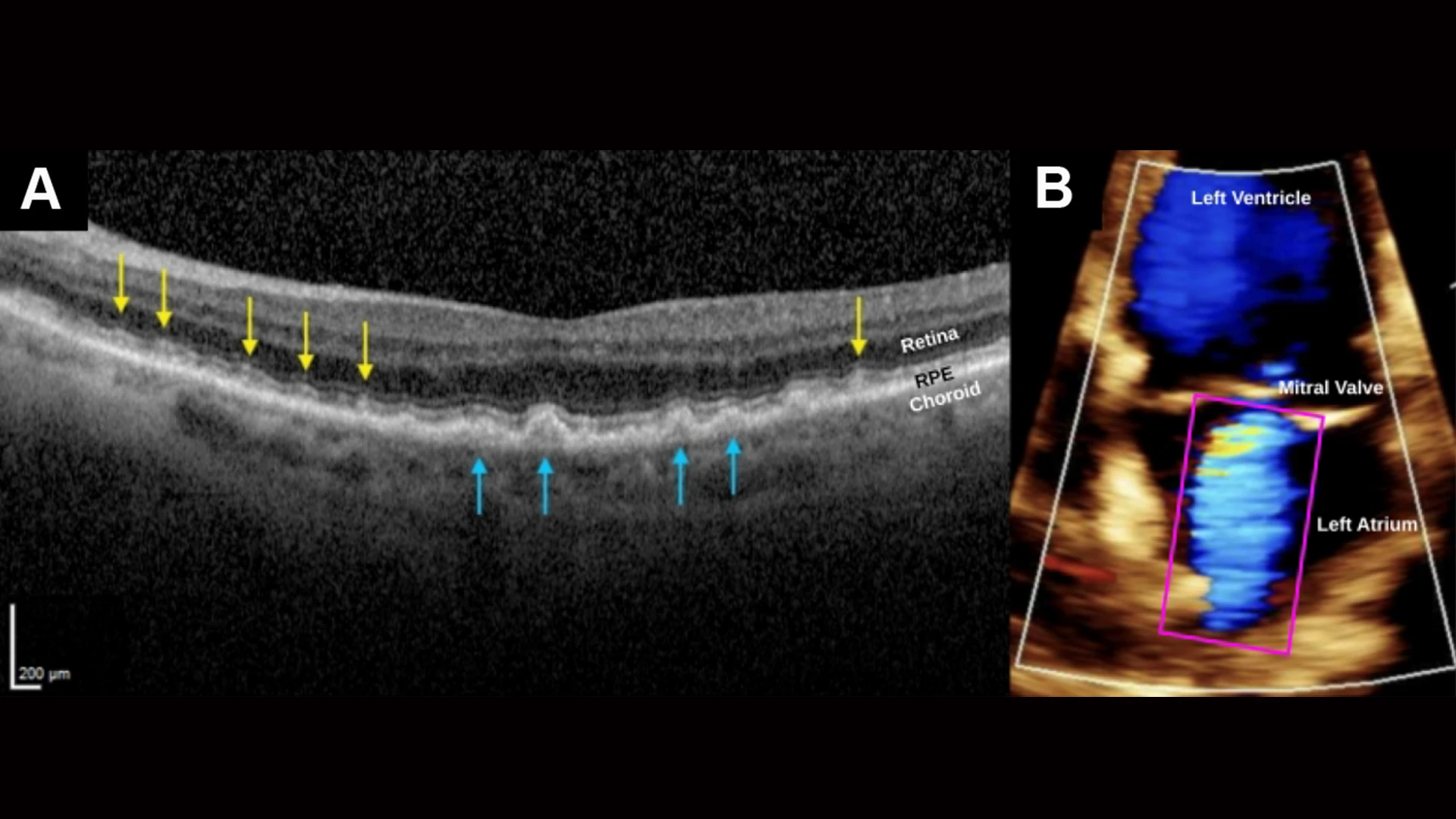
SDDs caused by severe cardiac valve disease. (A) SD-OCT scan, right eye, shows myriad SDDs (yellow arrows) above the RPE and several drusen (blue arrows) beneath the RPE in a patient with severe mitral valve regurgitation and compromised cardiac output. The insufficient ocular perfusion downstream then results in the SDDs. The left eye was similar. (B) A color doppler transthoracic echocardiogram of the left ventricle during systole, showing markedly reduced cardiac index, 1.87 L/min/m2, normal range 2.5-4.0, and severe mitral valve regurgitation. Left atrium shows turbid jet of arterial blood flowing retrograde from the left ventricle.
