Ophthalmologists have long agonized over how best to identify early warning signs of glaucoma, knowing they could provide the elusive key to diagnosing and derailing the progression to vision loss from the obstinate disease that afflicts more than 80 million people globally.
A new study from New York Eye and Ear Infirmary of Mount Sinai (NYEE) offers considerable hope. Researchers showed that measuring changes to flavoprotein fluorescence (FPF) in the optic nerve of people at various stages of glaucoma could set the stage for a critical new prognostic tool to potentially head off glaucoma before it can cause cell loss and structural damage to the optic nerve—damage that remains irreversible.
Mitochondria—the energy powerhouses of cells—produce FPF when they are stressed, and recent studies have suggested that dysfunction of mitochondrial proteins may be at the mechanistic root of glaucoma. Tellingly, FPF levels are elevated in people with glaucoma, especially in its early stages, and recede when mitochondrial oxidative stress is relieved by medication or surgery.
“The better we become at identifying early or ongoing degeneration to the optic nerve, the more proactive we can become at implementing protective therapy,” says Richard B. Rosen, MD, Vice Chair and Director of Ophthalmology Research at NYEE, and senior author of the study, published in the July/August 2022 issue of Ophthalmology Glaucoma. “Our study is the first to show that FPF may be an important objective measure for predicting glaucoma progression at an early stage, compared to measuring structural damage at the optic nerve head after it’s occurred.”
Mitochondrial oxidative stress is a key factor in many retinal degenerative diseases.
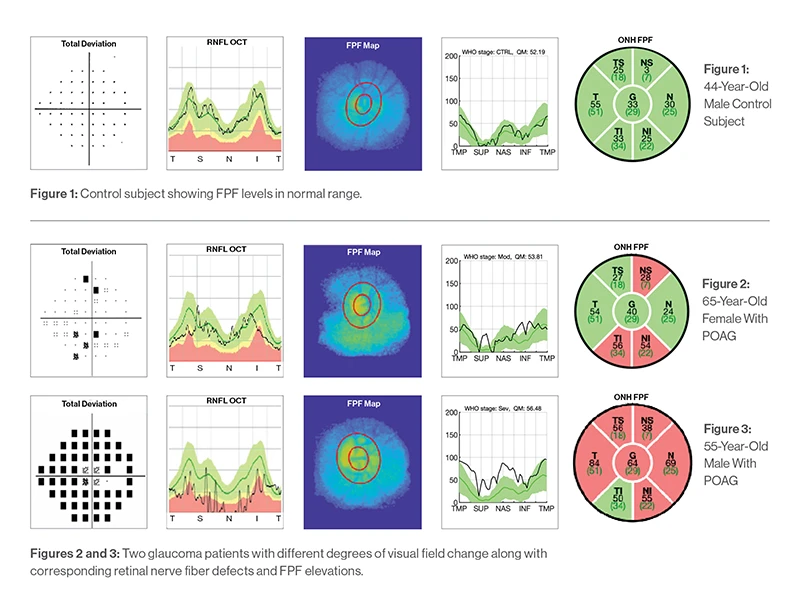
Figure 1: 44-year-old male control subject
Figure 2: 65-year-old female with POAG
Figure 3: 55-year-old male with POAG
Glaucoma is a group of optic neuropathies characterized by the degeneration and loss of retinal ganglion cells. Early diagnosis remains key to preventing progression and blindness since patients typically remain asymptomatic until late in the disease process, after irreversible damage has set in. Starting intervention early has been shown to improve treatment efficacy and long-term outcomes.
Flavoprotein fluorescence, for its part, could serve as an important biomarker and valuable supplement to intraocular pressure (IOP), retinal nerve fiber layer thickness, and visual field integrity, the standard parameters for detecting glaucoma. FPF could have particular utility for identifying disease status in patients without elevated IOP. This category includes the many patients with normal- or low-tension glaucoma who have pressures that fall within the normal range.
For their cross-sectional study, NYEE researchers used the OcuMet Beacon, a fundus camera with special filters that isolate fluorescence, to image and analyze 86 eyes, 50 with primary open-angle glaucoma and 36 with no disease.
“Our findings showed the presence of mitochondrial dysfunction at the optic nerve head rim of eyes with glaucomatous damage, and that the degree of FPF corresponds to disease severity,” explains Dr. Rosen. “That suggests that FPF could serve as a reliable metabolic marker of disease status. Moreover, because the technology we used provides a quick and noninvasive snapshot of the eye, it could potentially be a way to monitor the patient’s response to therapy over time, and to characterize the efficacy of neuroprotective therapies for glaucoma.”
While those observations are currently hypotheses, Dr. Rosen hopes to give them substance through his ongoing research with an even more advanced version of OcuMet Beacon, developed by OcuSciences, Inc.
“Through this imaging technique, we might be able to say to a patient, ‘Your FPF is up, so we need to add neuroprotective agents, or we may need to start you on therapies to reduce your pressure, even though it’s now considered normal,’” he elaborates. “This is a very exciting prospect, because for the first time, it would enable us to intervene before, and not after, the fact.”