Common across an array of corneal diseases is a little-understood phenomenon known as “crosstalk” that occurs between two organelles within the cells: the endoplasmic reticulum (ER) and the mitochondria. The way these components respond to intracellular stress could be pivotal to the death of corneal endothelial cells and a major contributor to such ocular pathologies as Fuchs’ endothelial corneal dystrophy (FECD), which affects 4 percent of the population in this country over 40, as well as age-related macular degeneration, retinitis pigmentosa, glaucoma, and diabetes retinopathy.
A team of scientists from the Mount Sinai/New York Eye and Ear (NYEE) Eye and Vision Research Institute is starting to shed valuable light on the pathophysiology of the ER-mitochondrial interaction, with implications for not just ocular but neurodegenerative, cardiovascular, and metabolic diseases where crosstalk has also been reported. In a study published in the November 2023 issue of Investigative Ophthalmology and Visual Science, the team described for the first time the three protein-signaling pathways that are activated when ER stress disrupts mitochondrial bioenergetics, resulting in corneal endothelial cell apoptosis.
“We’re moving the field ahead by demonstrating through our models the impact of endoplasmic reticulum stress on mitochondrial dysfunction, and how intraorganellar crosstalk between the two can mediate corneal stress levels,” says senior author Varun Kumar, PhD, Assistant Professor of Ophthalmology, and Pharmacological Sciences, at the Icahn School of Medicine at Mount Sinai. “We plan to screen drugs that target both the ER and the mitochondria, possibly through the inhibition of one of their pro-apoptotic pathways. This pathway has been studied in the past in other diseases, but limited studies about their crosstalk are available for ocular disorders.”
In a separate but related study, the Kumar lab is investigating the potential use of an existing Food and Drug Administration-approved drug for uveal-type disorders as an ER stress inhibitor, along with several other compounds in phase 1 clinical studies.
Fuchs’ endothelial corneal dystrophy is an age-related disorder with a higher incidence in females. Currently, it is attributed to oxidant-antioxidant imbalance in the cells, along with the independent contribution of mitochondrial or ER stress and genetic factors. The only treatment for FECD is corneal transplantation, a major surgical intervention.
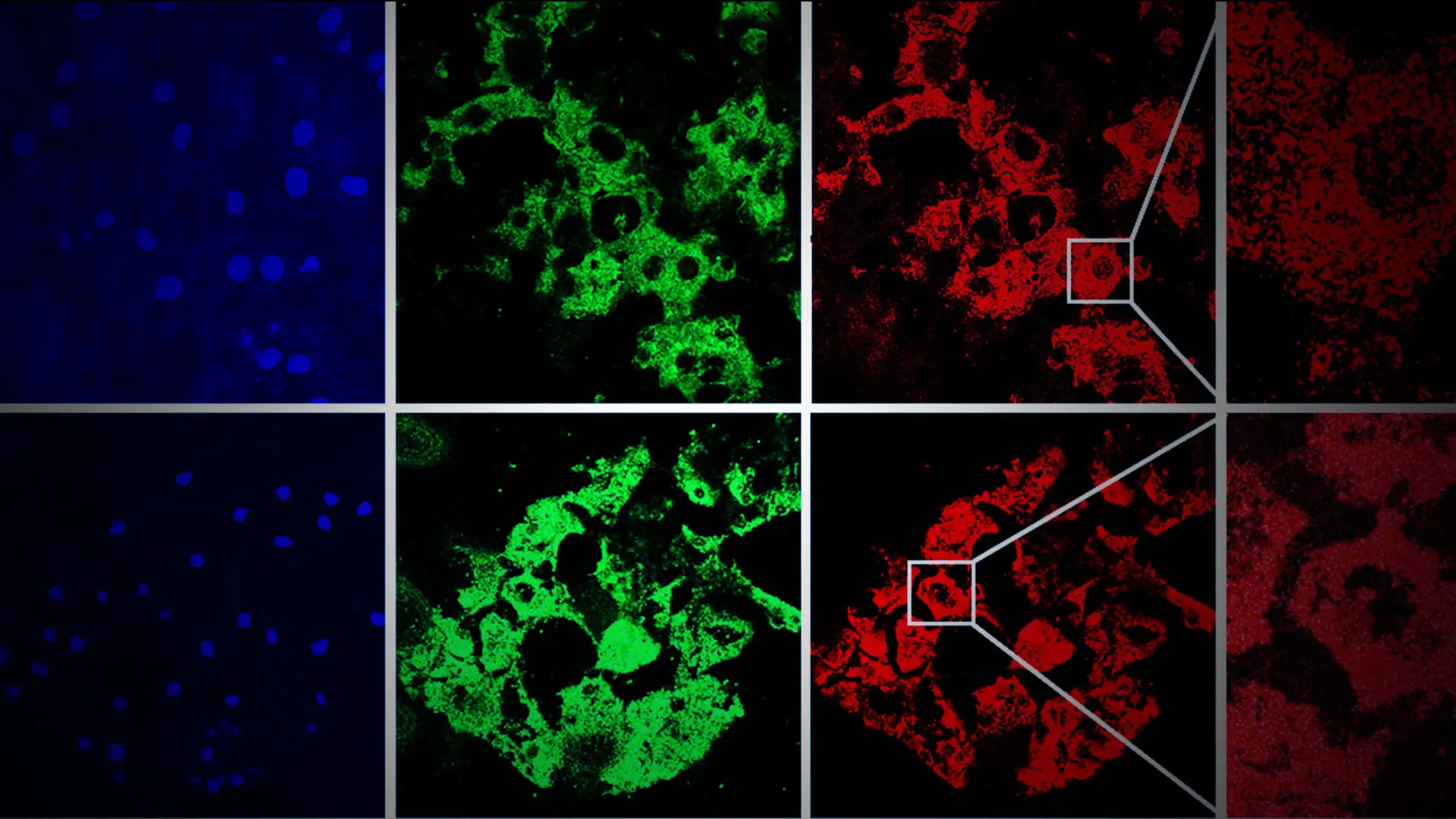
In their investigation, Mount Sinai/NYEE researchers learned that immortalized human corneal endothelial cell line F35T had significantly high induction of pro-apoptotic ER stress markers compared to normal control immortalized human corneal endothelial cell line HCEnC-21T. Treatment of HCEnC-21T with tunicamycin (an ER stress inducer) activated three major ER stress pathways (PERK-eIF2-CHOP, IRE1-XBP1, and ATF6), disrupted mitochondrial ATP production and oxygen consumption, and induced mitochondrial fragmentation. These effects on mitochondria contributed to corneal endothelial cell death, explains Dr. Kumar, whose interdisciplinary lab draws on a wide range of Mount Sinai resources, including tissue banking and high-throughput screening of potential drug candidates.
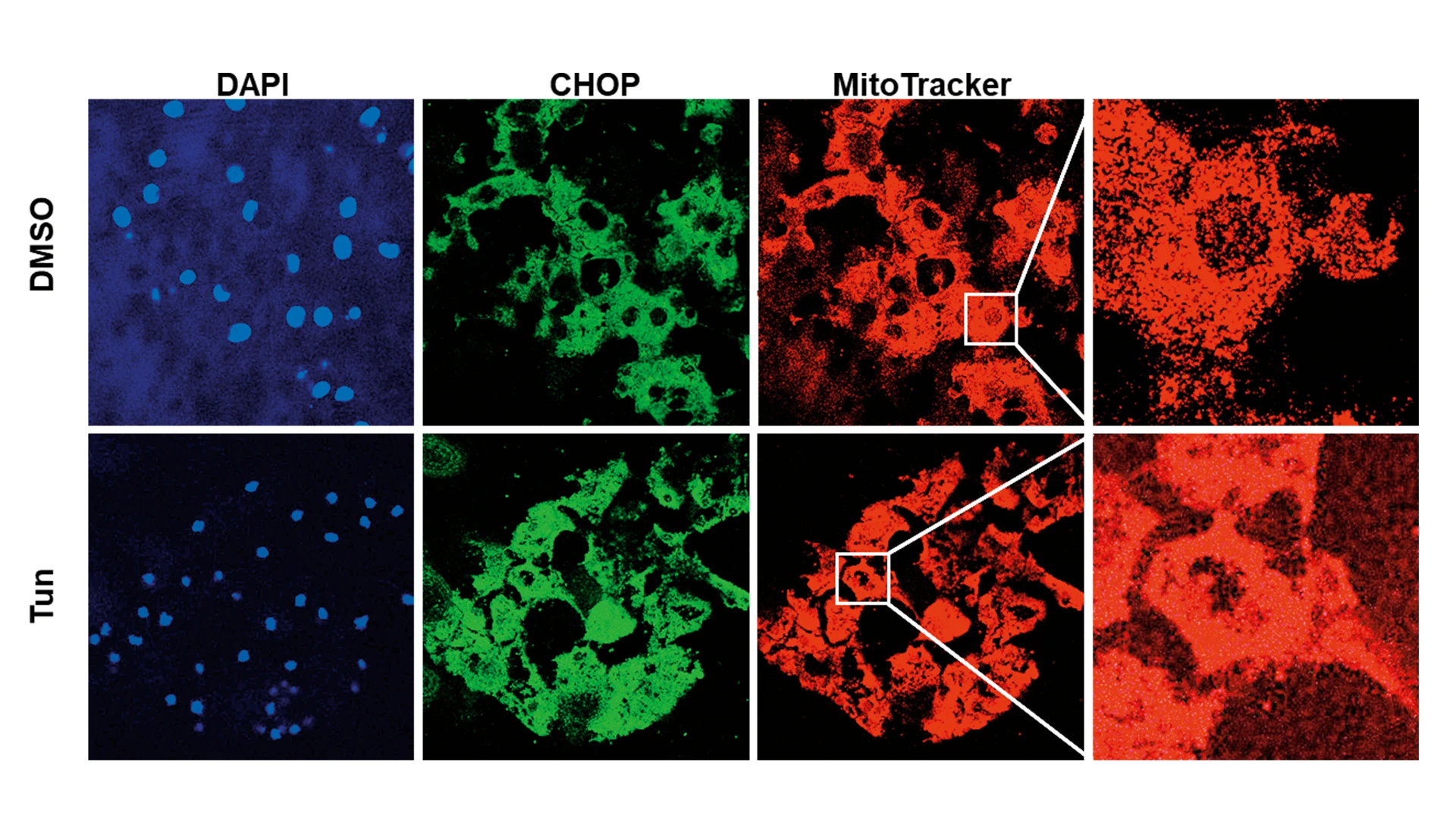
Figure demonstrating induction of pro-apoptotic ER stress protein, CHOP (green), and mitochondrial fragmentation (red), after tunicamycin (Tun) in human corneal endothelial tissues ex vivo.
Another promising way the vision science team is attempting to understand and eventually harness the mechanisms of ER-mitochondria crosstalk is through the proteins of both organelles, along with their signaling pathways. Specifically, they are examining the behavior of these proteins at the point of their connecting membranes—about which little is known by science—using animal models to isolate those structures for functional analysis.
“Focusing on these key membranes, which constitute more than half of the cellular content, is an exciting new direction for the field,” notes Dr. Kumar, who joined the Department of Ophthalmology in January 2022. “The hope is to use our findings to uncover new pharmacologic approaches to modulating the membranes—which would represent a huge advance for diseases like Fuchs’ dystrophy.”