Using breakthrough approaches to sequential imaging of retinal blood flow, New York Eye and Ear Infirmary of Mount Sinai (NYEE) has united the worlds of ophthalmology and hematology in a way that promises to yield significant benefits for more than 100,000 Americans, most of them of African descent, who suffer from sickle cell disease.
In a new study published in the July 2022 issue of Ophthalmology Science, researchers reported the use of dynamic optical coherence tomography angiography (OCTA) coupled with specially processed adaptive optics scanning light ophthalmoscopy (AOSLO) to reveal cellular phenomena of sickle cell capillary blood flow in extraordinary detail, opening the door to earlier diagnosis and individualized treatment.
The synergy of these two imaging modalities allowed the team from NYEE and the Department of Medicine at the Icahn School of Medicine at Mount Sinai to reveal the microscopic details of retinal vaso-occlusion dynamics in patients with sickle cell disease, enabling clinicians to evaluate for each individual the effectiveness of a new wave of therapeutics that have recently become available. That evidence includes intermittent blood flow within capillaries, which open and close momentarily—an early sign of impending vaso-occlusion, which can result in blindness if blood flow is completely shut off. Significantly, OCTA and AOSLO were shown by researchers to be sensitive enough to detect improved retinal perfusion in a sickle cell patient two months following initiation of oral hydroxyurea therapy.
“Despite its clinical advantages, OCTA is still limited in its ability to resolve the details of cellular flow and vessel wall features due to the optical aberrations of the eye,” explains senior author Toco Y. P. Chui, PhD, Director of the David E. Marrus Adaptive Optics Imaging Laboratory and creator of the software algorithm that allowed for the marriage of the two imaging platforms. “AOSLO compensates for these limitations through its ability to resolve retinal features such as red blood cells and vascular mural cells at a cellular level in vivo. For the first time, we’re able to watch sickle red blood cells flow through capillaries and visualize the mechanisms of vaso-occlusion.”
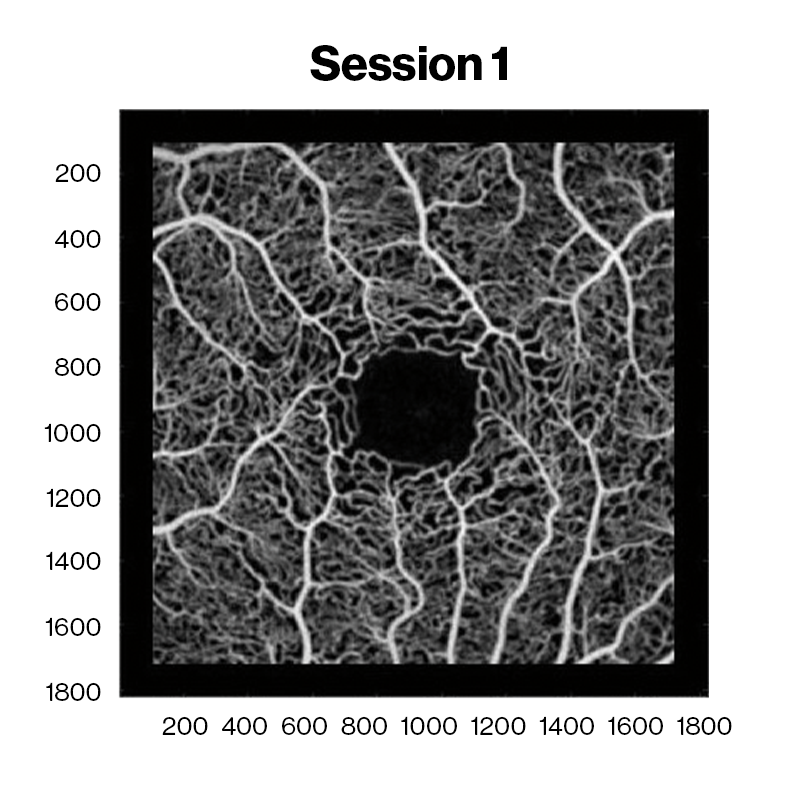
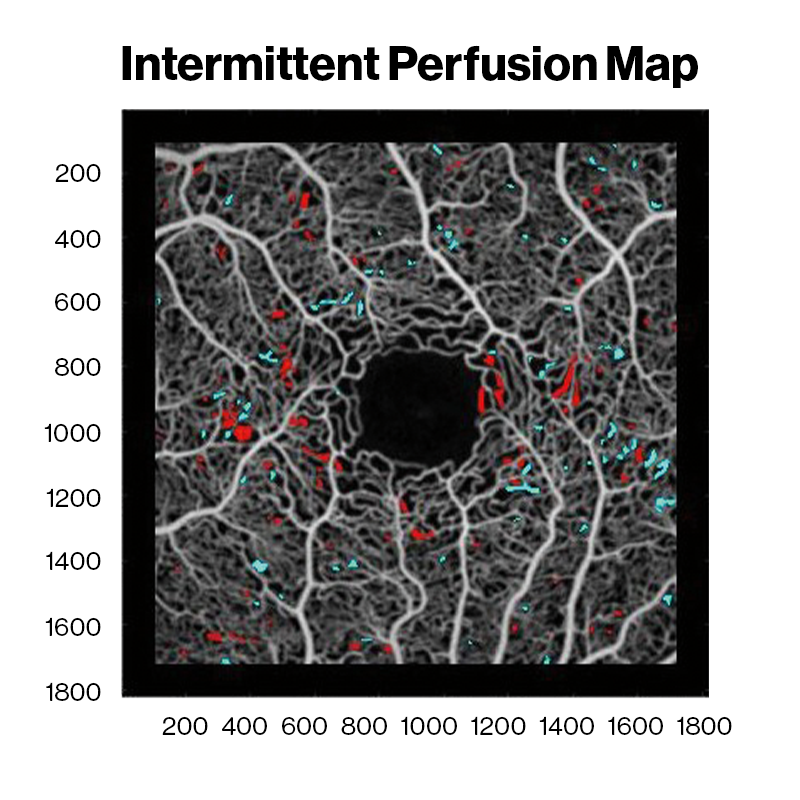
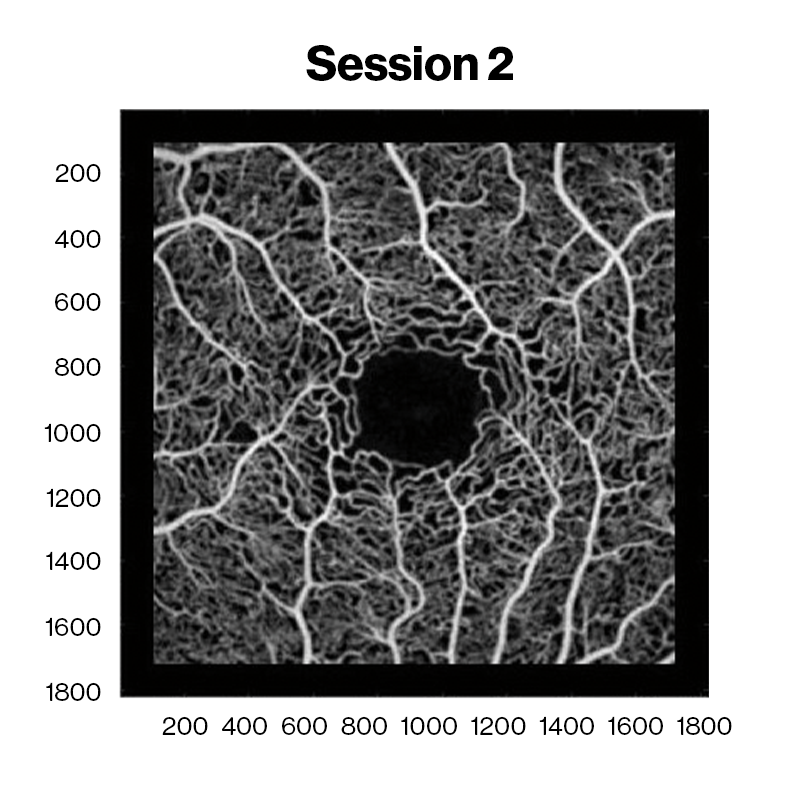
Averaged OCT angiography images of a sickle cell HbSC disease patient at David E. Marrus Adaptive Optics Imaging Laboratory. Red and cyan indicate capillary nonperfusion and reperfusion between sessions, respectively.
These discoveries are particularly exciting to Jeffrey Glassberg, MD, MA, a co-author of the study and Director of the Mount Sinai Comprehensive Sickle Cell Program, which treats hundreds of sickle cell patients at The Mount Sinai Hospital. “Our research is not only about identifying retinal disease, but more broadly about how sickle cells clump together in tiny blood vessels and can occlude blood flow,” he says. “The retina is really a disease-sensitive capillary bed that serves as a very sensitive proxy for the microvasculature status of other parts of the body, including the brain and kidneys.”
A major finding of this joint research, according to Dr. Glassberg, is that sickle cell disease is not always caused by red blood cells that are distorted into sickle shapes and stick to one another, but sometimes by fragments of cellular debris, which monocytes and macrophages are unable to scavenge and eliminate as part of their normal duties. “The discovery that cellular debris can completely overwhelm the blood circulatory system and impact the level of vaso-occlusion is completely new to the field of sickle cell disease,” Dr. Glassberg notes.
Building on the success of its imaging study, the NYEE/Mount Sinai team recently launched a new clinical investigation backed by a $4 million grant awarded by the National Institutes of Health for the next five years. That study plans to enroll and carefully track 200 patients to determine the effectiveness of the recently developed intermittent perfusion index—an algorithm-driven measure of what percentage of blood vessels in the retina are transiently blocked over the course of an hour, based on repeat or sequential imaging during that timeframe. By contrast, repeat scans of individuals without sickle cell retinopathy show continuous blood flow with few if any interruptions.
“The algorithm that Dr. Chui developed allows us to actually see how effective new treatments for sickle cell disease are for individual patients,” says Richard B. Rosen, MD, Chief of the Retina Service at Mount Sinai Health System and Vice Chair and Director of Ophthalmology Research at NYEE. “Our new study is an important step toward integrating this powerful imaging technology into clinical practice. It moves us closer to individualized medicine and could potentially help determine how new sickle cell drugs get approved.”
Featured

Toco Y. P. Chui, PhD
Associate Professor of Ophthalmology

Jeffrey Glassberg, MD, MA
Associate Professor of Emergency Medicine, and Medicine (Hematology and Medical Oncology)