Stem cell replacement therapy promises to usher in a new era in ophthalmic medicine, and nowhere are the hopes higher than with age-related macular degeneration (AMD), the leading cause of blindness, which affects more than 200,000 individuals worldwide. A number of human trials using induced pluripotent stem cells (iPSC) to replace lost retinal pigment epithelium (RPE) have shown encouraging results in restoring some vision, but they come with a complication: the production of epiretinal membranes.
A team of researchers at the Mount Sinai/New York Eye and Ear (NYEE) Eye and Vision Research Institute has discovered a potential way around this adverse event, which has impeded progress in the field of iPSC-RPE cell replacement. As reported in the August 2022 issue of Frontiers in Cell and Developmental Biology, the approach provides for inhibition of the p38 signaling pathway, minimizing the likelihood of epiretinal membrane formation as well as proliferative vitreoretinopathy, which is fueled by inflammation from the transplant surgery.
“We’ve found a way to improve cell stability with RPE transplantation that may reduce the occurrence of adverse events observed in the clinic,” says senior author Timothy Blenkinsop, PhD, Associate Professor of Cell, Developmental, and Regenerative Biology, and Ophthalmology, Icahn School of Medicine at Mount Sinai. “Our study could lead to new protocols for retinal transplantation using iPSCs, which, because of their self-renewal capability, are a highly promising source to generate unlimited RPE for cell therapy.”
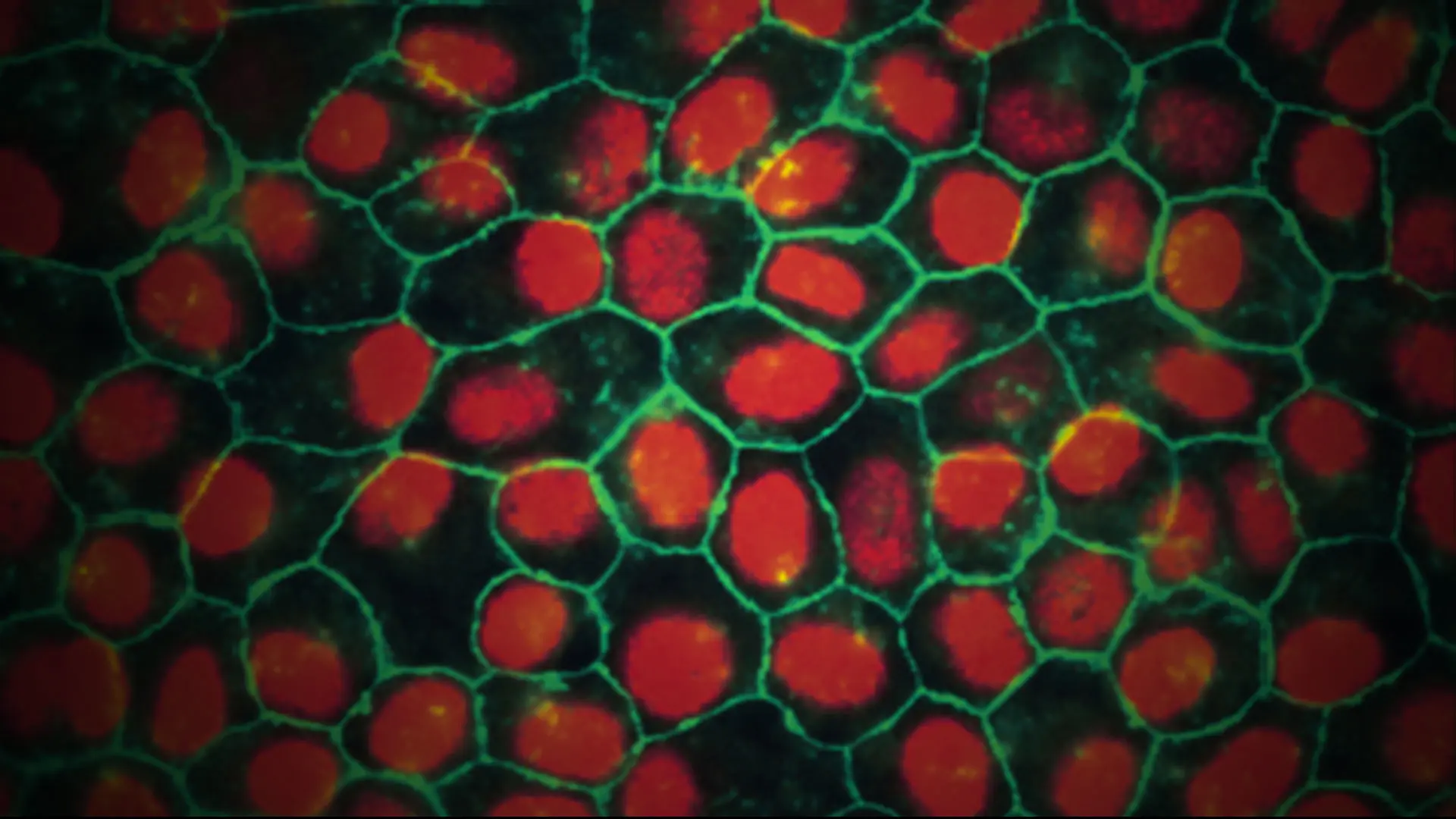
Postulating that the source of epiretinal membrane formation could be incomplete cell maturation, Dr. Blenkinsop and his team conducted a comparison of gene expression profiles between iPSC-RPE, adult primary RPE taken from the human eye, and RPE from the immortalized ARPE-19 cell line, a popular source for studying RPE biology. They found that iPSC and adult primary sources were comparable in their ability to express normal RPE genes, and did so at a higher level than the ARPE-19 cell line. What’s more, iPSC-RPE expressed networks involved in early eye development and muscle contraction. The downside, however, is the finding by researchers that iPSC-RPE exhibits an epigenetic plasticity, meaning a tendency to change its physiology and structural properties when placed in an inflammatory environment, such as stem cell transplantation surgery.
“We developed a model for our study that mimicked the inflammatory environment that induces proliferative vitreoretinopathy, and showed that iPSC-RPE changes its physiology to become less RPE-like and more muscle-like,” explains Dr. Blenkinsop. “Just as importantly, we discovered that p38 inhibition can repress production of these contractile membranes, potentially minimizing adverse events. Reducing the risk of epiretinal membrane formation would represent a major step forward for RPE cell replacement therapy.”
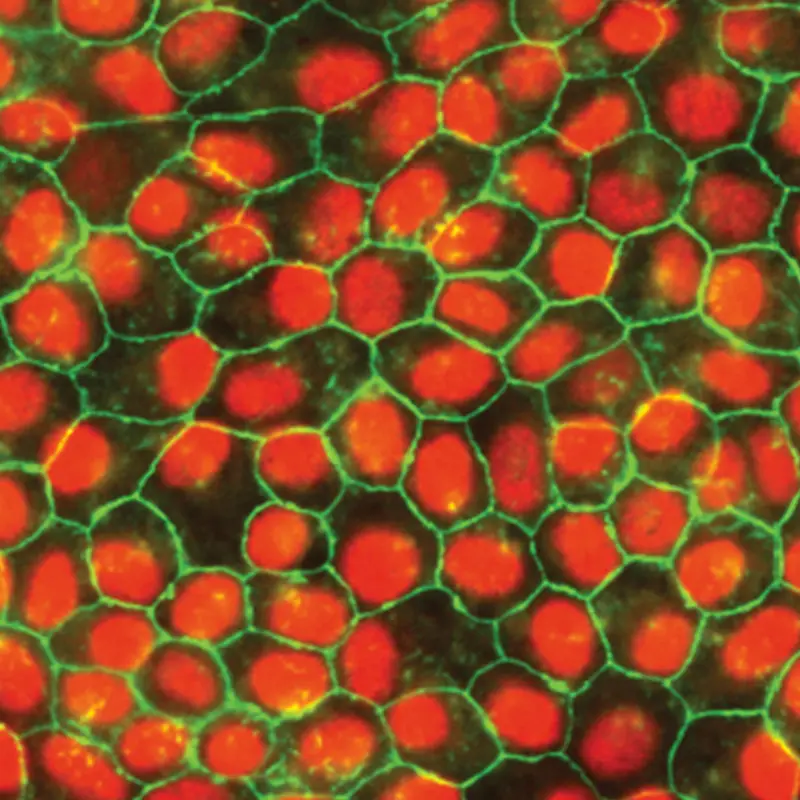
Pluripotent stem cell-derived retinal pigment epithelium exhibiting typical protein localization and morphology of native tissue
Featured

Timothy Blenkinsop, PhD
Associate Professor of Cell, Developmental and Regenerative BIology, and Ophthalmology