For at least 30 years, scientists have attempted to show an association between age-related macular degeneration (AMD), cardiovascular disease (CVD), and stroke, but concrete results have proven elusive. A new study led by a team from New York Eye and Ear Infirmary of Mount Sinai (NYEE) suggests the reason why: past investigations were focused on only a partial picture of the disease pathology.
According to the latest study, published in the July 2022 issue of RETINA, researchers detected, through retinal imaging of patients, two distinct disease pathways leading to advanced age-related macular degeneration. One is soft drusen beneath the retinal pigment epithelium (RPE), and the other is subretinal drusenoid deposits (SDDs) above the RPE. Each of these lipid-bearing deposits is tied to different sets of risk factors (including serum and genetic), but in only one pathway—SDDs—do they include cardiovascular disease and stroke. These findings could have significant public health implications in screening for macular degeneration, the leading cause of blindness, and cardiac disease and stroke, the leading causes of death in the developed world.
“Scientists couldn’t find the link between AMD and CVD and stroke in the past because they weren’t looking at the right population with AMD,” explains lead author R. Theodore Smith, MD, PhD, Professor of Ophthalmology, Icahn School of Medicine at Mount Sinai, and Director of Biomolecular Retinal Imaging, NYEE. “We learned that if OCT (optical coherence tomography) shows a patient has subretinal drusenoid deposits—which occur in about half of AMD cases—they’re at a much higher risk for life-threatening cardiovascular disease and stroke. At the same time, if cardiac imaging shows an irregularity, the patient is a much stronger candidate for AMD and should also be screened for that condition.”
Dr. Smith and his team believe the connective thread between AMD and CVD is vascular. Poor ocular circulation of blood not only results in subretinal drusenoid deposits but can also serve as an important marker for systemic vascular disease impacting the heart. “We now have a way to screen or study patients who have either macular degeneration or significant cardiac or neurovascular disease and find out who might also be harboring the other,” points out Dr. Smith. “This could provide a critical new diagnostic tool for identifying the millions of people who walk around not knowing they have a disorder that could be life-threatening or sight-threatening.”
To that end, Dr. Smith is working closely with Jagat Narula, MD, PhD, Philip J. and Harriet L. Goodhart Professor of Medicine and Director of the Cardiovascular Imaging Program at the Icahn School of Medicine at Mount Sinai, on collaborative research that spans both of their imaging fields. Specifically, NYEE is building on the cutting-edge diagnostic work of Dr. Narula and other Mount Sinai scientists using CT scanning and tracer isotopes to detect coronary artery disease. NYEE, for its part, employs gold- standard OCT scanning and even more advanced spectral domain OCT to detect subretinal drusenoid deposits above the retinal pigmented epithelium. A combination of these modalities will help to drive ongoing research designed to correlate cardiac disease and stroke with age-related macular degeneration.
Another player in that collaborative effort will be the Blavatnik Family Women’s Health Research Institute at Mount Sinai. Recognizing that coronary artery disease is an alarmingly neglected area of public health, the Institute is helping to develop research that will target women with high-risk vascular conditions that have been understudied in the past.
The latest discovery by NYEE and Mount Sinai researchers of dual AMD pathways will help to accelerate that study and others into the pathogenic mechanism of AMD. “By separating AMD into two different diseases, we stand a much better chance of figuring out what’s occurring at the cellular level of each,” observes Dr. Smith. “And that could potentially lead to new medications or treatments to improve the circulation or blood supply to the vessels of the eye, for example. Clearly, being able to apply our research on a population scale to vascular disease screening and treatment could benefit countless numbers of people worldwide.”
FIGURES:
Subretinal drusenoid deposits (SDDs) are driven by carotid stenosis, myocardial failure, and cardiac valvular dysfunction. Figures 1, 2, and 3 illustrate three major categories of vascular disease causing the SDD form of age-related macular degeneration (AMD), not soft drusen. Infrared reflectance scans are paired with corresponding optical coherence tomography (OCT) scans in standard format for retinal OCTs.
Fig. 1: Left-Sided Stroke and Internal Carotid Artery (ICA) Stenosis
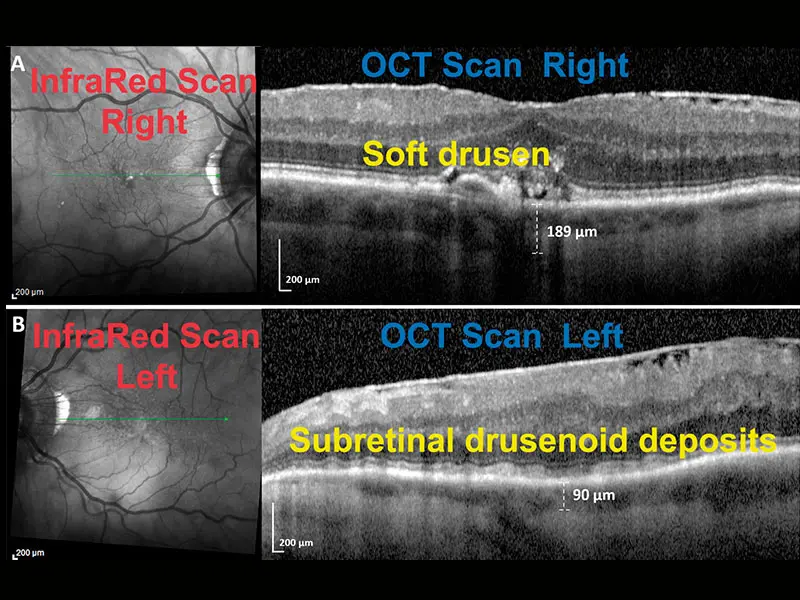
A: Right Eye
Infrared scan: Bright drusen centrally. Green line: Location of OCT scan. OCT scan: Confluent soft drusen centrally, under and elevating the bright retinal pigment epithelial (RPE) band. No SDD. Choroidal thickness, 189 microns.
B: Left Eye
Infrared scan: Multiple homogeneous dark lesions of SDD. Green line: Location of OCT scan. OCT scan: Smooth waves of SDD over the bright RPE and under the retina (stage 2). Choroidal thickness, 90 microns. SDDs and choroidal thinning are present only in the left eye, where ICA stenosis compromises ophthalmic artery and choroidal perfusion. The uncompromised right eye has no SDD and normal choroidal thickness, an experiment in nature showing that the carotid perfusion deficit precisely determines the SDDs.
Fig. 2: Myocardial Infarction (MI)
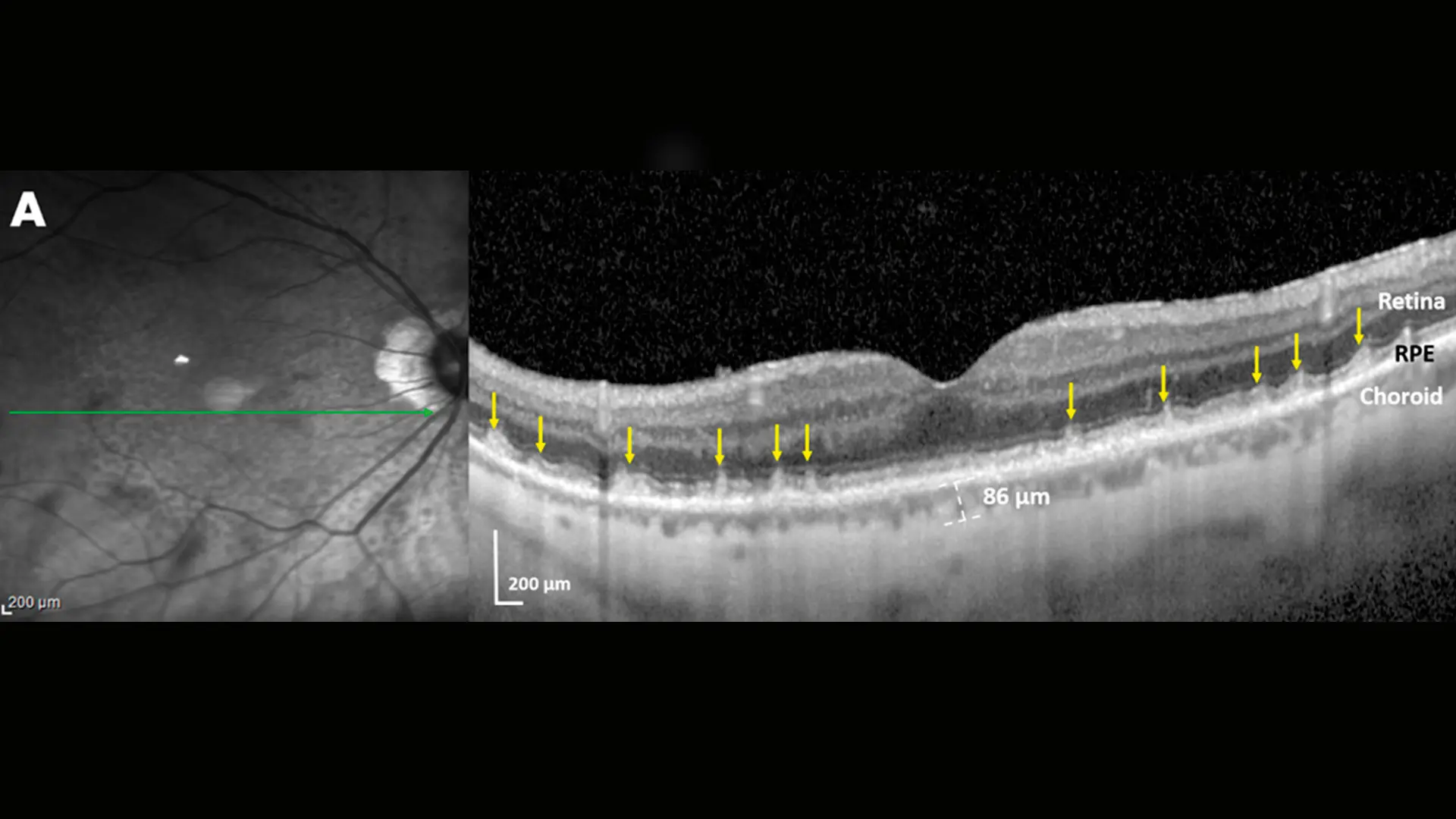
Right Eye
Infrared scan: Multiple homogeneous dark lesions of SDD. Green line: Location of OCT scan. OCT scan: Multiple SDD (yellow arrows) with a conical appearance above the RPE penetrating the overlying retina (stage 3). Choroidal thickness, 86 microns. Choroidal thinning is also due to the myopia, but myopia is not a cause of SDD. The SDD are caused by the poor systemic perfusion in the setting of MI. Left eye was similar.
Fig. 3: Severe Aortic Stenosis
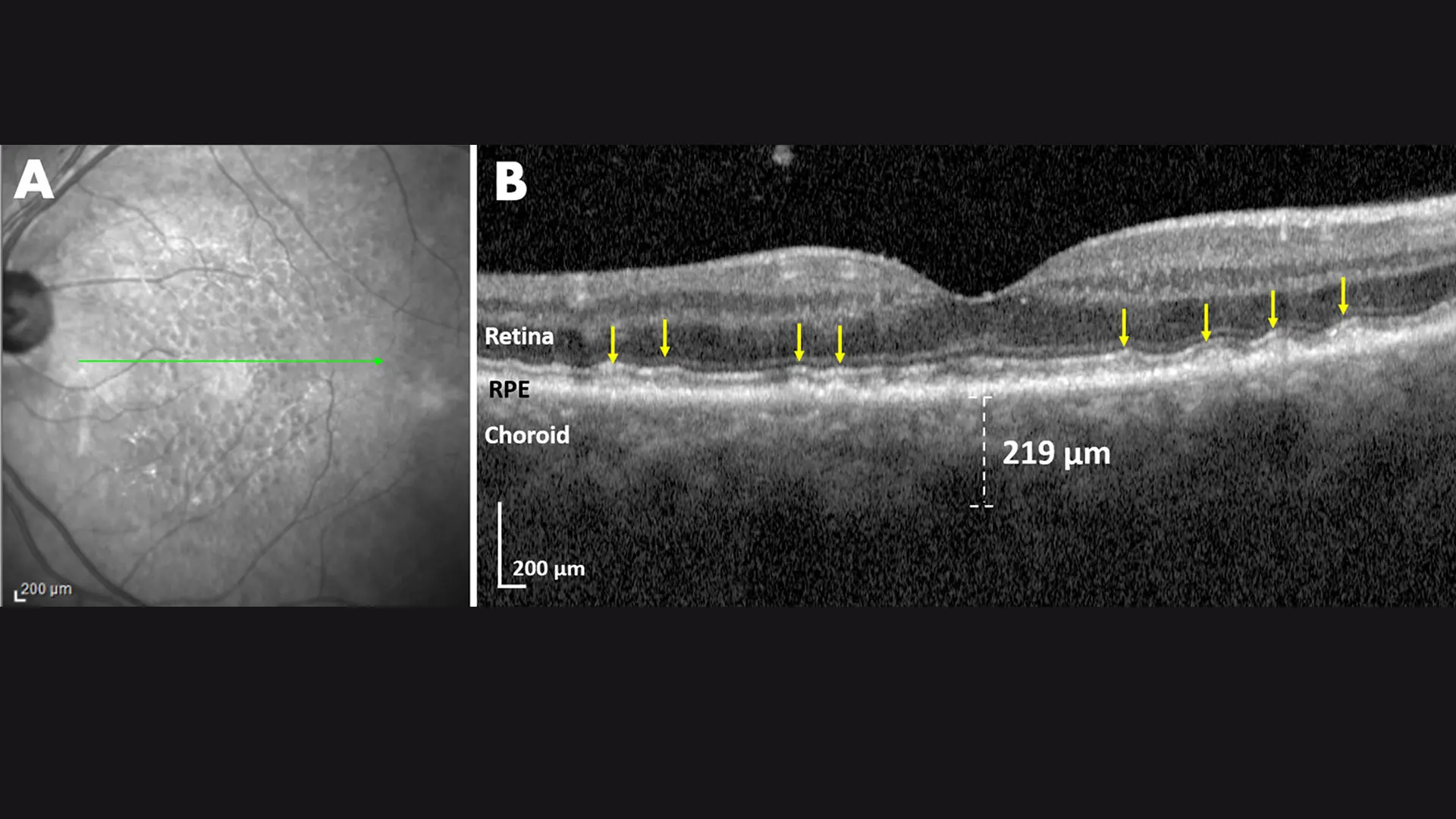
Left Eye
Infrared scan: Multiple homogeneous dark lesions of SDD. Green line: Location of OCT scan. OCT scan: Multiple SDD (yellow arrows) in several smooth, wave-like series. The measured choroidal thickness of 219 microns corrected for hyperopic refractive error is ~169 microns, more typical for SDD. The right eye had been lost to glaucoma and vascular disease. Aortic stenosis is an example of a cardiovascular disease unrelated to atherosclerosis, but compromising cardiac output and ophthalmic perfusion, hence causing SDDs.
Featured

R. Theodore Smith, MD, PhD
Professor of Ophthalmology